COVID-19 mRNA vaccines contain excessive amounts of bacterial DNA: evidence and implications
Recent studies by Kevin McKernan, a leading expert in DNA and RNA sequencing methods, have revealed that batches of the modified mRNA vaccines produced by both Pfizer and Moderna contain a high proportion of contaminating bacterial DNA. In total, DNA accounts for up to 20-35% of the nucleic acids contained in each of the vaccine lots. These alarmingly high concentrations far exceed levels considered safe by standards organizations such as the European Medicines Agency (EMA). This paper summarizes the evidence of this DNA contamination and discusses the possible health risks it poses to vaccine recipients.
1. The role of DNA in mRNA vaccine manufacturing
1.1. General Background
Most readers will be aware that
- the synthetic RNAs contained in the COVID-19 mRNA vaccines encode the SARS-CoV-2 spike protein;
- in living mammalian cells, the instructions for building a given protein molecule are stored as a gene within the DNA inside the nucleus;
- to build a given protein molecule, the cell first transcribes its gene into RNA and modifies the two ends of this molecule to form messenger RNA (mRNA). The mRNA is then transported from the nucleus to the cytoplasm, where it induces the cell’s protein factories – the ribosomes – to translatethe nucleotide sequence of the mRNA to the corresponding amino acid sequence and assemble the protein.
1.2. steps in mRNA vaccine manufacturing
Since the spike protein is a large molecule, so is the mRNA that encodes it. Total chemical synthesis of large mRNA molecules is not practical at scale. Therefore, to obtain the peak coding mRNA molecule, the process by which cells produce their own mRNAs is mimicked in vitro. This involves the following steps:
- A DNA copy of the gene for the spike protein is inserted into a bacterial plasmid. This is a ring-shaped, double-stranded DNA molecule that can exist in a bacterial cell independently of the cell’s own chromosomal DNA, and that can also be copied and transmitted to both daughter cells when that cell divides.
- The recombinant (artificial) plasmid carrying the spike protein gene is introduced into a cell of the bacterial species Escherichia coli(E. coli ). Since E. coli cells divide very rapidly, this single cell can, in a short time, grow into a very large number of cells. Each of these progenitor cells will contain its own inherited copies of the plasmid and thus the spike protein gene. Although there is a certain probability that the plasmid will be lost from some of the offspring during successive cell divisions, we can enforce its maintenance by giving it a selectable marker, which ensures that only the cells that retain the plasmid will survive. With the plasmids used by both Pfizer and Moderna, this selectable marker is a gene that gives host cells resistance to the antibiotic kanamycin. To apply selection, the bacteria are simply grown in the presence of the kanamycin.
- After growing a sufficient number of bacterial cells in a nutrient broth containing kanamycin, these cells are broken down and plasmid DNA is purified from the other components of the bacterial cells.
- The ring-shaped plasmid molecules are converted to linear form using a restriction enzyme, which cleaves both strands of the DNA molecule at a specific, unique site downstream of the spike protein gene. This step is necessary to prevent the formation of RNA molecules that are too long and can have undesirable effects in vivo. The linearized DNA molecules can be purified from the remaining circular molecules, but how and how efficiently this can be done in the production of the Pfizer and Modern vaccines is not publicly known.
- An RNA polymerase is used, in the presence of the necessary nucleoside building blocks and cofactors, to copy the spike protein gene from the DNA version on the linearized plasmid to the mRNA version. Both Pfizer and Moderna employ the T7 RNA polymerase, which is derived from the eponymous bacteriophage. This enzyme binds to a cognate promoter sequence, also derived from T7, which has been engineered into the plasmid upstream of the gene for the spike protein. This interaction between the polymerase and the promoter initiates transcription. At this stage, the synthetic nucleoside N-methyl-pseudouridine (mψU) is incorporated into the artificial RNA instead of the natural uridine nucleoside. When administered in vaccine form, RNA modified in this way is less stimulating to the innate immune system than RNA containing the natural uridine. It also translates more effectively into protein, and under certain conditions more resistant to degradation[1]. Both Pfizer and Moderna’s mRNA vaccines contain mψU instead of uridine.
- The two ends of each RNA molecule are enzymatically coupled to certain moieties that are also found at these positions within natural mammalian mRNAs, and that enhance their biological activity and stability in vivo.
These steps provide a functional mRNA capable of instructing the ribosomes of the cells to produce the spike protein. However, at this stage, the product is not yet pure – all bacterially derived template DNA is still present. The latter should not be included in the final drug product, because it poses health risks to recipients (see section 4). To get rid of this DNA, another enzyme called DNase is added. This should break down the DNA into smaller fragments, which can then be removed from the much larger RNA molecules by filtration and other purification techniques. In the final step, the mRNA is combined with a lipid mixture in order to pack it into lipid nanoparticles (LNPs), which induce human cells to take up the mRNA molecule and make the spike protein.
2. What did we previously know about the problem of DNA contamination?
In short, very little. The FDA evaluation reports on both vaccines[2,3] do not mention the issue at all. The European Medicines Agency (EMA) evaluation report on the Pfizer vaccine mentions that “The robustness of the DNase digestion step is not considered thoroughly demonstrated”[4, p. 17]. Similar language is used in the EMA report on the Moderna vaccine[5, p. 19f]. However, based on this scant information alone, it is impossible to say whether the problem was considered serious, and what remedies were required by the regulator, if any.
3. Independent evidence on DNA contamination of mRNA products
As of April 3, 2023, Kevin McKernan described his recent findings in three articles on his Subtack website[6-8]. The experiments described in the first two reports were performed on “bivalent” vaccine samples recently introduced by Pfizer and Moderna. These preparations resemble the previous “monovalent” ones in their chemical composition, i.e. they should contain highly pure mRNA, complexed with a mixture of lipid (fat-like) molecules in mRNA/lipid nanoparticles. The only difference between the two varieties is that bivalent vaccines contain a mixture of two mRNAs encoding two antigenic variants of the spike protein. This has no bearing on the technical problem of DNA contamination as such. However, we note that the extent of DNA contamination can vary between production batches, and that so far only a small number of batches have been characterized in this regard.
3.1. McKernan’s first report
In an early study[6], McKernan characterized both the RNA and DNA contained in mRNA vaccines.
3.1.1 Extraction and direct characterization of vaccine nucleic acids
The first step was to remove the lipids in order to obtain the pure nucleic acids. The solvent-based method used does not discriminate between DNA and RNA – if both are present, both will be recovered. The extracted nucleic acids were separated according to size. This revealed not only the expected regular, full-peak mRNA species, but also smaller fragments, which had previously been noted both by regulators and in published work by one of the manufacturers[9]. More surprisingly, RNA species larger than the full-length mRNA were also found. These species remain uncharacterized.
3.1.2 Amplification of the extracted nucleic acids
As a preparatory step to determine the exact nucleotide sequences of the extracted nucleic acids, they were amplified by PCR methods. In the case of RNA, PCR was preceded by reverse transcription into DNA using a dedicated enzyme (reverse transcriptase). Since the main purpose of this study was to study RNA and not DNA, this amplification step was biased against DNA by adding actinomycin D, which under the given experimental conditions selectively inhibits DNA synthesis. Thus, relatively low amounts of DNA were recovered in the amplified sample. However, in the case of the Pfizer vaccine, the amount of DNA determined to be present already exceeded the limit arbitrarily decided by the EMA for the maximum allowable ratio of DNA to RNA.
3.1.3 DNA sequencing results
With the Pfizer and Moderna products, DNA sequences of complete DNA plasmids were obtained, although some ambiguity remained in the case of the Moderna plasmids. The characteristics of the plasmid sequences will therefore be discussed in connection with McKernan’s second study, which used more and more pure DNA for sequencing and therefore provided more reliable results.
3.2. McKernan’s second report
The second study[7] focused on quantifying and characterizing the DNA contamination that was qualitatively detected in the first.
3.2.1. plasmid DNA contained in mRNA vaccines is competent to propagate in bacterial cells
In the first experiment, it was determined whether the plasmid DNA whose presence had been inferred from the previous sequence results is indeed biologically functional, in that it can be introduced and persist within bacterial cells. For this purpose, nucleic acids were again extracted from the vaccine samples. These nucleic acids were mixed with a suspension of E. coli cells that had been made competent for DNA uptake.
After inducing these cells to take up the DNA and giving them some time to recover, they were spread in Petri dishes filled with a solidified growth medium containing kanamycin. As noted earlier, kanamycin will kill any E. coli cell that does not contain a resistance gene to it. Therefore, the observed growth of bacterial colonies in these Petri dishes confirmed that some cells had indeed acquired resistance to kanamycin by taking up and spreading the plasmids. This was observed with both the Pfizer and Modern vaccine samples.
In this context, we should note that only circular, but not linearized, plasmid molecules can be efficiently introduced into bacterial cells. The success of this experiment therefore suggests that some of the plasmid molecules escaped the linearization step (step 4 in section 1.2) and went through the entire production process in the circular form that exists in bacterial cells. On the other hand, since the number of bacterial colonies observed in this experiment was not high, it is likely that most of the DNA had indeed been linearized. Since the biological hazards of foreign DNA within our own bodies can vary depending on whether it is linear or circular, the likely presence of both forms in vaccines is noteworthy. The exact proportions of circular and linear DNA in the mixtures are yet to be determined.
3.2.2 The abundance of contaminating DNA
The second major finding of this study is the PCR quantification of both the DNA and mRNA contained in the vaccine samples. As you may know, in a PCR reaction, a chosen segment of a nucleic acid sequence is reduced by enzymatic synthesis over several successive reaction cycles. From the number of cycles (or duplications) required to reach a certain threshold concentration, we can calculate how many copies of the target sequence were present at the start.
In these experiments, the experimental format chosen was multiplex PCR, that is, two target sequences were amplified in a single reaction mixture. One of these targets was within the spike protein gene, and thus should be present in both the plasmid DNA molecules and the spike mRNA molecules transcribed from them. In order to include the mRNA molecules in this amplification, PCR was again preceded by reverse transcription.
The other target sequence was within the kanamycin resistance gene, which should only be present in the plasmid DNA. By comparing the number of cycles required for each of the two targets to cross the threshold, it was determined that up to 35% of the total nucleic acid contained in the vaccines is actually DNA. For comparison, the EMA has stipulated that DNA should be no more than 0.033% of the total nucleic acids.
3.2.3 Determination of plasmid DNA sequences
The plasmids that had originally been contained in the vaccines and then introduced into bacterial cells (see section 3.2.1) were again isolated from these bacterial cultures, and their complete DNA sequences were determined. Such sequences were provided in full in McKernan’s first study,[6] but he indicated that he was still working on corroborating and refining the sequencing data. Meanwhile, the functional characteristics of the plasmid DNA found in the Pfizer vaccine samples are shown in Figure 1. They will be discussed in connection with the risk assessment.
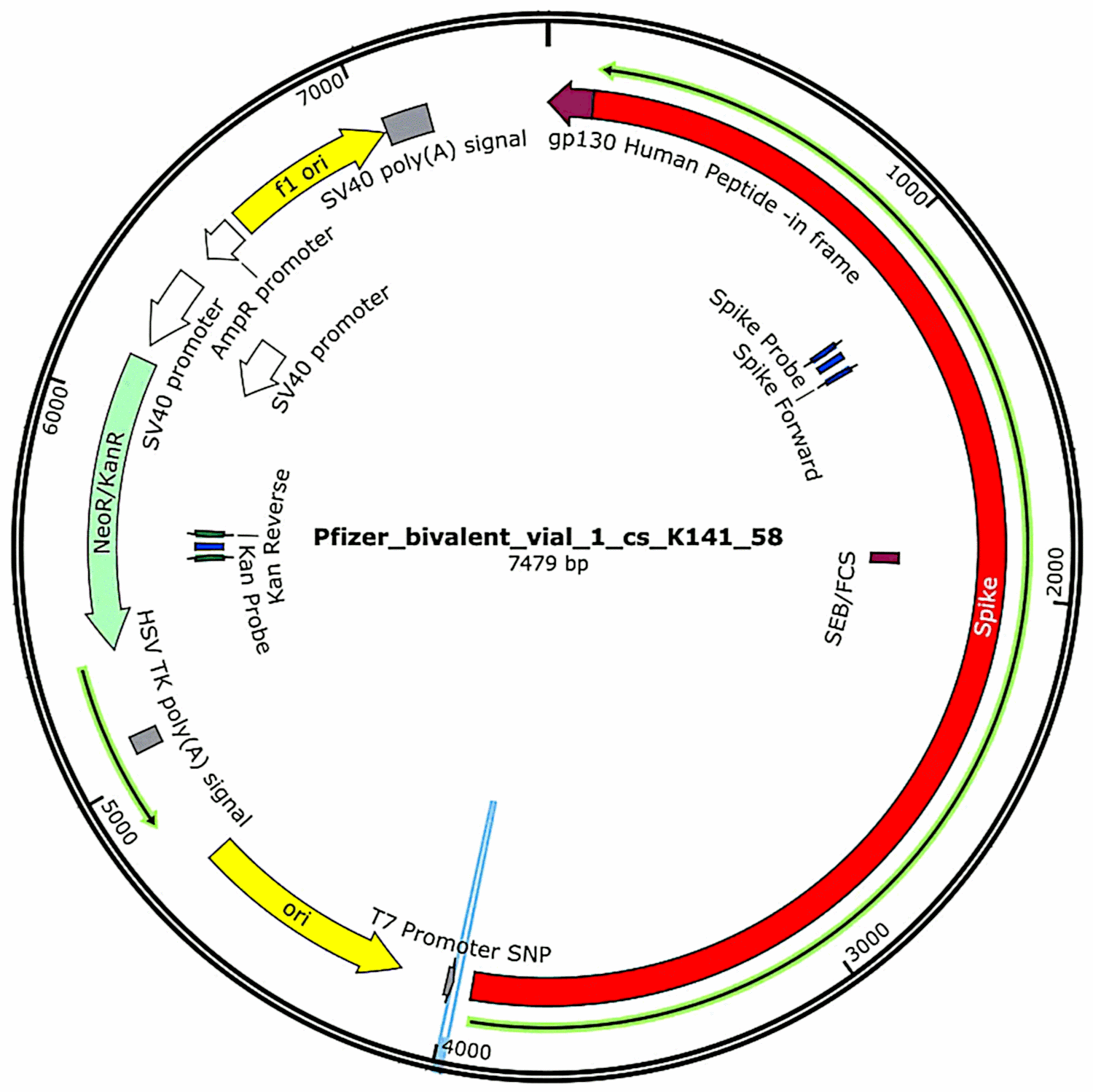
3.3. McKernan’s third report
In his most recent report to date, McKernan examined eight vials from an earlier batch of the Pfizer vaccine using the quantitative PCR method described above. The DNA content in this case was markedly lower than in the bivalent vaccine samples, but still exceeded the MPE limit by a factor of 18-70[8].
4. Risk assessment
We must assume that the recombinant DNA found in mRNA vaccines can be introduced into the cells of our body, and that this will be aided by lipid nanoparticles, just as with the mRNA itself. This poses several different types of health risks.
4.1. prolongation of the duration of spike protein expression
A key argument that is regularly used to promote the perceived safety of the mRNA vaccine is that the mRNA is short-lived in vivo, and that the expression of the encoded antigen will therefore be short-lived as well. For example, the EMA evaluation report on the Pfizer vaccine states in relation to animal experiments on a model vaccine that were accepted in lieu of adequate studies on the COVID-19 vaccine itself[4, p. 46]:
As expected with an mRNA product, luciferase expression was transient … The signal slowly decreased during the first 72 hours and after 6 and 9 days the signals were further weakened to levels approximately 18- and 7-fold higher than the signals obtained from animals injected with buffered control.
These results seem to agree with two in vitro studies that compared the duration of protein expression between messenger RNA species that were identical in sequence but contained uridine or mψU, respectively; as noted earlier, the latter is also present in Pfizer and Modern mRNA vaccines. In both studies[1,10], the mψU-modified RNA species induced significantly higher levels of protein expression, however, this high expression decreased with a half-life similar to that of the unmodified RNA. None of the half-lives that can be inferred from the data in either study exceed 4.5 days.
It is, however, clear from multiple studies on vaccinated individuals that both the spike protein itself and the nucleic acids encoding it can be detected, in the bloodstream and in various organs, for weeks and even months after injection[11-15]. This discrepancy between in vitro and in vivo studies has so far been difficult to understand. The high levels of residual plasmid DNA in the vaccines detected by McKernan now suggest a plausible explanation.
For bacterial plasmid DNA to support prolonged spike protein expression, two conditions must be met:
- the plasmid DNA must persist within the cells of our body, and
- the spike protein gene in that plasmid must be transcribed into mRNA by our own cellular RNA polymerase II.
Although we do not yet have any direct experimental data on Pfizer and Modern spike expression plasmids, precedent suggests that, in fact, both requirements are met. Recombinant plasmids expressing clotting factor IX have been found to persist in the liver cells of experimental animals at stable levels for up to 1.5 years,[16,17] which corresponded to the total duration of the experiment. One might object that the plasmids used in these studies were circular, whereas most plasmid DNA contained in mRNA vaccines is probably in linear form (see section 1.2). In response, we note that, first, some circular plasmid DNA probably remains (see Section 3.2.1), and, second, that recombinant viral DNA has been shown to persist in linear form within animals for similarly long periods of time[18], suggesting that the same may be true of plasmid DNA.
In the studies cited[16,17], the gene encoding the protein of interest (factor IX) had been under the control of a mammalian promoter, and indeed the factor IX protein was expressed at stable levels throughout. In contrast, the spike protein gene contained in the Pfizer and Moderna expression plasmids is under the control of a T7 bacteriophage promoter. We cannot assume a priori that this promoter will function in the absence of its cognate T7 RNA polymerase. It has, however, been experimentally confirmed that, in fact, the T7 promoter also binds cellular RNA polymerase II and causes protein expression in mammalian cells[19].
In summary, the possibility that the observed long-lasting expression of the spike protein is caused by the plasmid DNA contained in the mRNA vaccines should be taken seriously. The prolonged persistence of spike protein mRNA and its expression after vaccination, detected in biopsies and autopsies, has been unequivocally associated with severe damage[14,20], which is most likely mediated by an immune attack on the cells expressing this foreign antigen. The omission of corresponding experimental studies in the preclinical testing phase, together with the scale of this contamination, creates a totally unacceptable safety risk.
4.2. risks associated with regulatory SV40-derived DNA sequences
One feature that was identified by McKernan in the Pfizer plasmids but not in the Moderna expression plasmids[6] is a promoter derived from the SV40 virus, which belongs to the polyoma family (see section 4.2). This promoter is located upstream of the kanamycin resistance gene; and since it is active in mammalian cells, the protein encoded by this resistance gene will be expressed in any cell containing this DNA. Like the spike protein, this protein is a foreign antigen, so it too can trigger an immune attack on the cells that express it.
The SV40 promoter also includes an internal replication origin that can potentially cause copies of the plasmid within mammalian cells[21]. This will require the presence of the large viral T antigen, a protein that directly recognizes this origin and then initiates replication of the DNA molecule. This protein is not encoded by the plasmid, nor is it normally present in our body cells, but can be provided either by the SV40 virus itself or by a related polyoma virus. A minority of the human population is latently infected with SV40, and this latent infection is associated with some malignant and non-malignant diseases[22]. If a copy of the Pfizer plasmid is taken into a cell that harbors SV40, then additional copies of it can be effectively formed.
Two related polyoma viruses that are much more widespread in the human population are BK and JC virus[23,24]. The JC large T antigen is apparently less effective in conjunction with the SV40 origin than the SV40 protein itself[25], but replication of the Pfizer plasmid in cells latently infected with JC or BK viruses cannot, however, be ruled out. Additional copies of the plasmid generated in this way would amplify all the other risks discussed in this section, with the possible exception of non-specific inflammation (see section 4.4).
4.3. genomic insertion of plasmid DNA
The scenarios discussed so far all involve the episodic independent persistence of plasmid DNA; it will be present near the chromosomes (within the cell nucleus), but will not have become an integral part of any of them. Such independent, non-replicating plasmid molecules will tend to be lost during cell division[26]. However, as we will see, in some cases, a plasmid molecule can actually be integrated into one of the chromosomes of its host cell, and will then be inherited by all the descendants of that cell.
Chromosome integration is a form of “genotoxicity”, i.e. toxicity that causes genetic damage. Regarding the possibility of such effects, the EMA evaluation report on the Pfizer mRNA vaccine succinctly notes[4, p. 50]:
No genotoxicity studies were provided. This is acceptable since the components of the vaccine formulation are lipids and RNA that are not expected to have genotoxic potential.
Apparently, the EMA experts were assuming that RNA in general will not affect the integrity of the host cell genome. This view is incorrect, and the first evidence to prove it recently celebrated its fiftieth anniversary[27]. However, the detection of copious amounts of plasmid DNA in both manufacturers’ vaccines now obviates the need to make that case. Certainly even the EMA scientists will be aware that this DNA can be integrated into the genome of human host cells. No specific sequence features are required for such integration to occur and consequently it has been observed in the same way with mammalian virus DNA, bacteriophages, and plasmids[28]. It is worth noting that such insertions can occur at random locations in the genome, but genes that are being actively expressed by the cell are more often affected[29].
The stable chromosomal integration of a bacterial plasmid into the chromosomal DNA of mammalian cells was demonstrated as early as 1982[30]. The plasmid in question shares multiple characteristics with those used in the production of Moderna and Pfizer’s mRNA vaccines. The introduction of foreign or modified genes into mammalian cells using this and similar techniques has since become commonplace in experimental research and biotechnology. The methodology is referred to as transfection, and the organisms modified in this way as transgenes. We note that stable integration can occur with both linear and circular plasmid DNA[31].
In this context, we should also consider the previously published study by Aldén et al.[32], who detected DNA copies of the spike protein gene in a human liver cell line after these cells were exposed to Pfizer mRNA vaccine. Based on the assumption that the vaccine contained essentially pure mRNA but not DNA, they took this observation as evidence that the synthetic mRNA had undergone reverse transcription within these cells. Their interpretation is plausible, because such reverse transcription is known to occur in principle and has been previously reported in cells from patients infected with the SARS-CoV-2 virus[33]. However, in light of McKernan’s discovery that Pfizer vaccine vials can contain substantial amounts of DNA, it seems equally possible that the observations of Aldén et al. simply indicated cellular uptake of this DNA. In any case, however, their findings indicate the presence of spike-coding DNA within these cells, which indicates a risk of genomic insertion.
4.3.1 Genomic insertion in gene therapy using retroviral vectors
In proper gene therapy, chromosomal integration is often desired, since it will correct the genetic defect in question in a lasting way. For this purpose, special DNA vectors have been developed that have a much higher propensity to undergo such integration. These vectors are derived from retroviruses, whose complete survival strategy is based on genomic integration. It turns out, however, that integration, when it occurs in the wrong place within the genome, often induces malignant diseases, especially leukemia[34]. This is in fact so common that it has prevented the widespread adoption of gene therapy, even in diseases for which all other therapeutic options are equally fraught with very serious risks. A good example is adenosine deaminase deficiency, a metabolic disease that eliminates lymphocytes, thus causing severe combined immune deficiency (SCID), a condition that without treatment is always fatal during childhood. This disease is in principle a very suitable target for gene therapy, but a bone marrow transplant from a matched and related donor remains the preferred therapeutic option because of the severe risk of gene therapy-induced malignancies[35].
4.3.2 How does genomic insertion cause malignancies?
Our genome contains multiple genes that can give rise to cancer if their expression level – the rate at which mRNA and protein molecules are synthesized from them – is too low or too high. A foreign DNA molecule may happen to insert directly into such a gene and knock it out completely, or it may insert next to it, and a strong promoter present in that foreign DNA may cause overexpression of the gene in question. In addition, it has been observed that insertion events can also cause genome-wide changes in DNA methylation, which will affect the expression levels of many genes; and some of these changes may contribute to the induction of malignancy. It is important to note that this effect has been observed not only in viral DNA, but also in bacterial plasmids[36].
When cells are isolated from a healthy human or animal organ and grown in cell culture, they divide for a limited number of generations and then die. In contrast, cells derived from malignant tumors and leukemias can be propagated indefinitely. A similar change can also occur in cultured cells, which thus become immortalized and typically also lose some characteristics that are characteristic of their tissue of origin. This transformation can be induced, for example, by infecting the cells with the aforementioned SV40 virus. Similarly, cells can be transformed by transfection with a plasmid derived from SV40 that retains the crucial parts of the viral genome, including the gene encoding the large T antigen. On the other hand, if the large T antigen is absent from the plasmid, transformation does not typically occur[30]. However, some exceptions have been reported[37,38]. These cases must have arisen from the disruption or downregulation of cellular genes involved in proliferation control.
4.3.3 Genomic integration into germline cells
Oocytes can be transfected in vivo at certain stages of maturation[39], as can sperm-producing cells within the testes[40]. In the latter case, the offspring of animals subjected to such treatment have been shown to be transgenic. It cannot therefore be ruled out that people injected with mRNA vaccines that also contain DNA will subsequently give rise to transgenic children. The insertion of DNA into germ cells may also interfere with early intrauterine development and thus induce spontaneous abortions or malformations.
4.3.4 How should we assess the risk of genomic insertion?
It is certainly true that bacterial plasmids have a lower propensity for insertion into our chromosomal DNA than gene therapy vectors specially designed for efficient integration. But exactly how great is the risk in the case of plasmids contained in mRNA vaccines? The simple answer is that no one knows. This is not because it is unknown in principle, but because the appropriate experimental studies in animals, and subsequently in humans, have not been done; or if they have, the results have been withheld from the public, and apparently also from regulators.
How would such risks be assessed in properly conducted approval procedures? Current FDA guidance on the testing and approval of gene therapies[41] recommends that, in the clinical testing phase, patients be monitored for 15 full years after administration, with annual examinations during the initial five years. This applies to the vectors for which the chromosomal insertion is intended. The guidance document proceeds to construct a false dichotomy between insertion and non-insertion vectors, but the dividing line between them remains blurred. On the one hand, the guidance document suggests that
GT [gene therapy] products that are based on vectors such as plasmids … are not prone to integrate or reactivate after latency, generally have a lower risk of delayed adverse events,
but, on the other hand, states that
changes in the methods used to introduce plasmid DNA vectors into cells … result in higher integration frequencies (Ref. 27).
The reference cited in this last quote is a study by Wang et al.[42], who unequivocally identified plasmid DNA insertion in vivo after intramuscular injection, which was followed by electroporation. Although electroporation increased cellular uptake of the injected DNA relative to injection of “naked” DNA alone, it was probably much less effective in this regard than the lipid nanoparticles contained in mRNA vaccines. Consequently, we should expect some chromosomal integration of the contaminating plasmid DNA in vivo.
4.4. pro-inflammatory effect of bacterial DNA
The human innate immune system reacts with inflammation to various bacterial macromolecules, including DNA. The large amounts of DNA present in vaccines should be considered as contributing to inflammation near the injection site, and potentially also elsewhere in the body.
5. Conclusion
The presence of contaminating plasmid DNA in Pfizer and Moderna’s mRNA vaccines entails serious health risks, in addition to those that were already known and understood. These risks include prolonged expression of the spike protein, which can lead to corresponding and more destructive autoimmune inflammation, and the induction of malignant disease following chromosomal integration of the plasmid DNA. In addition, the scale of contamination proves conclusively that manufacturers have not mastered or properly implemented the designed production processes. Each of these issues alone would be sufficient reason to require the immediate recall of these vaccines.
Acknowledgements
We thank Kevin McKernan and Ulrike Kämmerer for corrections and discussion.
Copyright
This text is licensed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0). This means that you are free to copy and reuse the content, provided that the original authors are credited. If you make changes to the text, you must explicitly indicate this. For more details, see the Creative Commons website[43].
References
- Andries, O. et al. (2015) N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-{}incorporated mRNA, providing enhanced protein expression and reducing immunogenicity in mammalian and mouse cell lines. J. Control.Release 217:337-344
- Anonymous, (2020) FDA briefing document: Pfizer-BioNTech COVID-19 vaccine.
- Anonymous, (2020) FDA briefing document : modern MRNA-1273.
- Anonymous, (2021) EMA assessment report: Comirnaty.
- Anonymous, (2021) EMA assessment report: COVID-19 Moderna Vaccine.
- McKernan, K. (2023) Deep sequencing of Moderna and Pfizer bivalent vaccines identifies contamination of expression vectors designed for plasmid amplification in bacteria.
- McKernan, K. (2023) Pfizer and Moderna bivalent vaccines contain 20-35% expression vectors and are competent at transforming E.coli.
- McKernan, K. (2023) DNA contamination in 8 vials of monovalent Pfizer mRNA vaccines.
- Patel, H.K. et al. (2023) Characterization of BNT162b2 mRNA to Evaluate Risk of Off-Target Antigen Translation. J. Pharm.Sci. DOI:10.1016/j.xphs.2023.01.007
- Pardi, N. et al. (2018) Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215:1571-1588
- Bansal, S. et al. (2021) Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism of Immune Activation by mRNA Vaccines. J. Immunol. 207:2405-2410
- Castruita, J.A.S. et al. (2023) SARS-CoV-2 spike RNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS 131:128-132
- Fertig, T.E. et al. (2022) Vaccine mRNA can be detected in blood at 15 days post-vaccination. Biomedicines 10:1538
- Magen, E. et al. (2022) Clinical and Molecular Characterization of a Rare Case of BNT162b2 mRNA COVID-19 Vaccine-Associated Myositis. Vaccines 10
- Röltgen, K. et al. (2022) Immune imprinting, amplitude of variant recognition and germinal center response in human SARS-CoV-2 infection and vaccination. Cell DOI:10.1016/j.cell.2022.01.018
- Miao, C.H. et al. (2001) Long-term and therapeutic-level hepatic gene expression of human factor IX after naked plasmid transfer in vivo. Mol. Ther. 3:947-57
- Ye, X. et al. (2003) Complete and sustained phenotypic correction of hemophilia B in mice after hepatic gene transfer of a high expression human factor IX plasmid. J. Thromb. Haemost. 1:103-11
- Jager, L. and Ehrhardt, A. (2009) Persistence of high-throughput adenoviral vectors as monomeric replication-defective genomes in vitro and in murine liver. Hum.Gene Ther. 20:883-96
- Li, Y.Q. et al. (2000) [The function of T7 promoter as cis-acting elements for polymerase II in eukaryotic cells]. Yi Chuan Xue Bao 27:455-61
- Mörz, M. (2022) A Case Report: Necrotizing Encephalitis and Multifocal Myocarditis after BNT162b2 mRNA Vaccination against Covid-19. Vaccines 10:2022060308
- Byrne, B.J. et al. (1983) Definition of the simian virus 40 early promoter region and demonstration of a host range bias in the enhancement effect of the simian virus 40 72-base-pair repeat. Proc. Natl.Acad.Sci. U. S. A. 80:721-5
- Rotondo, J.C. et al. (2019) Association Between Simian Virus 40 and Human Tumors. Front.Oncol. 9:670
- DeCaprio, J.A. and Garcea, R.L. (2013) A cornucopia of human polyomaviruses. Nat.Rev. Microbiol. 11:264-76
- Hussain, I. et al. (2020) Human BK and JC polyomaviruses: molecular insights and prevalence in Asia. Virus Res. 278:197860
- La Bella, F. and Ozer, H.L. (1985) Differential replication of SV40 and polyoma DNAs in Chinese hamster ovary cells. Virus Res. 2:329-44
- Ehrhardt, A. et al. (2003) Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J. Virol. 77:7689-95
- Baltimore, D. (1970) RNA-dependent DNA polymerase in RNA tumor virus virions. Nature 226:1209-11
- Doerfler, W. (2016) Beware of genome manipulations: epigenetic destabilization via (foreign) DNA insertions. Epigenomics 8:587-91
- Doerfler, W. (1996) A new concept in (adenoviral) oncogenesis: foreign DNA integration and its consequences. Biochim.Biophys.Acta 1288:F79-99
- Southern, P.J. and Berg, P. (1982) Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J. Mol. Appl. Genet. 1:327-41
- Stuchbury, G. and Münch, G. (2010) Optimizing the generation of stable neuronal cell lines by pre-transfection restriction enzyme digestion of plasmid DNA. Cytotechnology 62:189-94
- Aldén, M. et al. (2022) Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr.Emite Mol.Biol. 44:1115-1126
- Zhang, L. et al. (2020) SARS-CoV-2 RNA transcribed and integrated into the human genome. bioRxiv DOI:10.1101/2020.12.12.422516
- Staal, F.J.T. et al. (2008) Sola dosis facit venenum. Leukemia in gene therapy trials: a question of vectors, inserts and dosage? Leukemia 22:1849-1852
- Kohn, D.B. and Gaspar, H.B. (2017) How We Manage Adenosine Deaminase-Deficient Severe Combined Immune Deficiency (ADA SCID). J. Clin. Immunol. -n/a
- Doerfler, W. et al. (2018) Inheritable epigenetic response towards foreign DNA entry by mammalian host cells: a guardian of genomic stability. Epigenetics 13:1141-1153
- Sipehia, R. and Martucci, G. (1995) High-efficiency transformation of human endothelial cells by Apo E-mediated transfection with plasmid DNA. Biochem. Biophys.Commun. Res. 214:206-11
- Takahashi, M. et al. (2002) Transformation of MC3T3-E1 cells after stress and transfection with plasmid pSV2neo. Anticancer Res. 22:585-98
- Laurema, A. et al. (2003) Transfection of oocytes and other types of ovarian cells in rabbits after direct injection into uterine arteries of adenoviruses and plasmid/liposomes. Gene Ther. 10:580-4
- Dhup, S. and Majumdar, S.S. (2008) Transgenesis through permanent gene integration in spermatogonial cell repopulation in vivo. Nat.Methods 5:601-3
- Anonymous, (2020) Long-term Follow-Up after administration of human gene therapy products: Guidance for Industry.
- Wang, Z. et al. (2004) Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Therapy. 11:711-21
- Anonymous, (2023) Creative Commons Attribution 4.0 International License (CC BY 4.0).
Originally published in Doctors for Covid Ethics
Suggest a correction







