LEAKED LETTER from EMA Head to MEPs shows agency’s abject failure

Recently, I was given a leaked 8-page letter (dated April 20th) from the European Medicines Agency’s executive director (and former Big Pharma lobbyist) Emer Cooke to the Chair of COVID Special Committee, MEP Kathleen Van Brempt. I am sure it will be published soon on the EMA’s website in their ‘commitment to transparency’, no doubt.
Emer Cooke’s letter can be downloaded here.
Her letter is in response to further questions asked by various MEPs, such as, Robert Roos, Christine Anderson, Francesca Donator and Cristian Terhes regarding the COVID-19 mRNA vaccines brought up at the Special Committee on COVID-19 pandemic sessions.
I’ve previously written about Roos’ salient questions to Emer Cooke at the March 27 Special Committee hearing on COVID-19 and refuted the many unscientific untruths she recited, amongst her umming and erring.
Ms Cooke’s rambling 8-page written letter is no different.
It begins with a whitewashing of the nonclinical biodistribution study’s alarming data published by the Therapeutics Goods Administration (TGA) of Australia in January 2021 (around the start of the mRNA vaccine rollout). It glaringly omits the fact that the lipid nanoparticles (two of the four lipid compounds are completely novel and highly toxic and inflammatory) accumulated in the ovaries of female rats.
At least, her letter, “acknowledged that the lipid nanoparticles can distribute rather non-specifically to several organs such as liver, spleen, heart, kidney, lung and brain, with the liver appearing to be the organ where the lipid nanoparticles distribute most.”
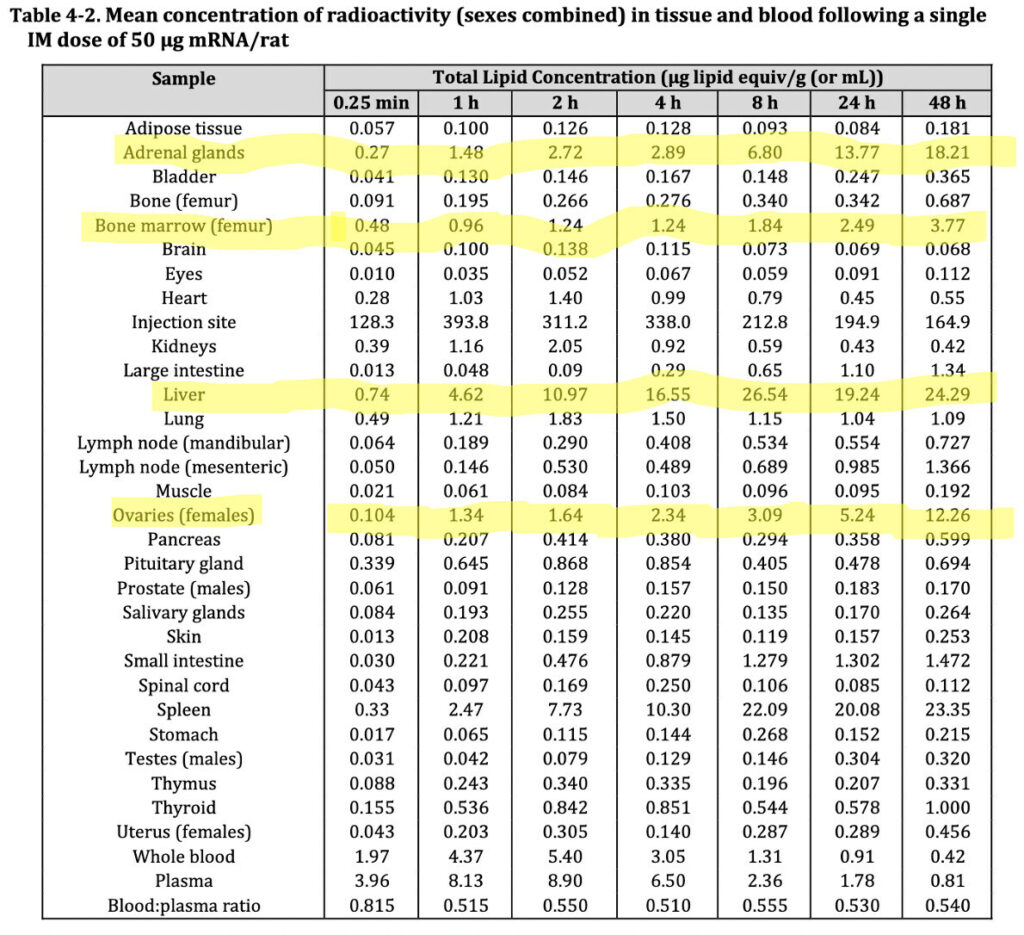
Her deliberate use of the term “rather non-specifically” is an obvious attempt to obfuscate the fact that these LNPs (lipid nanoparticles) accumulated in nearly every organ tissue, as shown in the table above, sourced from TGA’s nonclinical evaluation report– not shown in her letter.
She mentions the nonclinical repeat-dose and biodistribution (pharmacokinetic) studies “which indicates that a broader biodistribution is not a safety concern.”
However, she omits the fact that the biodistribution (pharmacokinetic) study results were not validated, not done according to GLP (Good Laboratory Practice) and did not study the distribution of the actual modRNA, the one in the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) but instead a surrogate luciferase modRNA was used. (See screenshot below of leaked Rapporteur’s Rolling Review assessment report from November 2020)
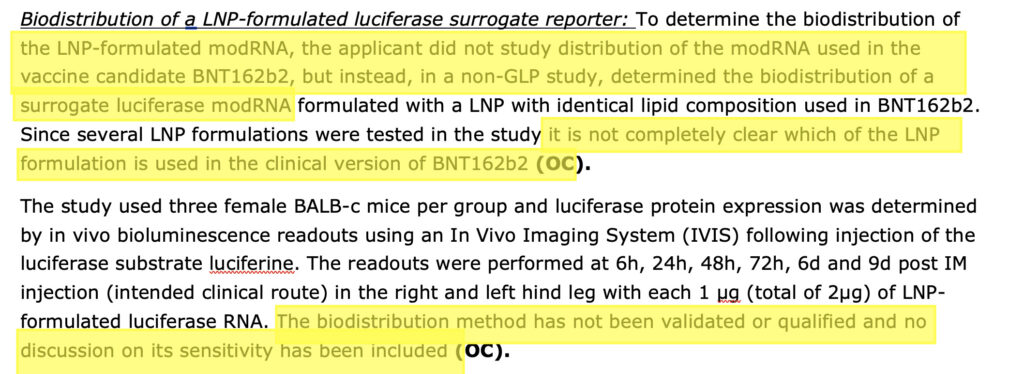
Shockingly, there were no nonclinical (in animals) studies done on the biodistribution of the actual modRNA encoding for the SARS-CoV-2 vaccinal spike protein, before it was injected into humans! A massive assumption has been made by all the regulators that the modRNA encoding for the vaccinal spike protein will be distributed around the body in exactly the same way as luciferase.
She writes in her 20 April 2023 letter, “the available evidence shows the amount of mRNA distributed to body organs is very small and is degraded within 6-9 days after injection.”
Just to reiterate, the two biodistribution studies in animals (by the way in one study, R-20-0072, only 3 mice were used!) were never tested on the actual modified mRNA used in the vaccine.
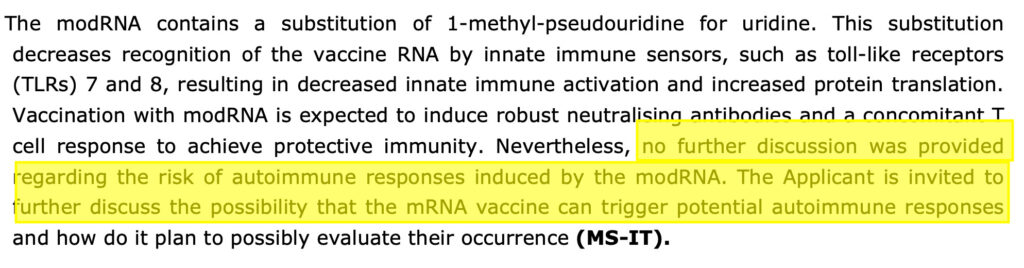
The modified mRNA in the Pfizer-BioNTech vaccine is synthetic. Every uridine has been replaced with N1-Methylpseudourine to evade the body’s innate immune response and promote protein translation. It does not break down quickly because it’s not natural and there are many safety problems arising from this fact, for example a triggering of auto-immune diseases.
The European Medicines Agency had this on their radar from November 2020.
I have also written extensively on the leaked EMA emails and Pfizer documents (Part 1 and Part 2) from November 2020, which alarmingly showed that the mRNA integrity level dropped down to 55% in the commercial batches of the vaccine compared to the clinical ones ~78%. RNA level is measure of how intact the RNA transcript is- the lower the level, the higher the concentration of truncated or fragmented RNA species (not intact). Key regulators were all aware of this issue but went ahead and authorised it anyway by accepting the lowering of the standard of the RNA integrity down to 50%! Therefore, allowing up to half of the RNA molecules in the vaccine to be truncated. Of course, none of this was mentioned in her letter.
This leads to my coverage of the #Blotgate scandal (Part 1 and Part 2) showing evidence that BioNTech falsified their Western blot assays (technique used to identify specific proteins) which they uses to ‘prove’ to the EMA and other regulators that the vaccine was comparable across different batches and that only the spike protein was being expressed by the vaccinal mRNA.
The EMA assessors were concerned that these truncated mRNA species could have the potential to express proteins other than the intended spike protein and wanted them to be further characterised. They also wanted the potential for them to “cause an autoimmune process” to be evaluated by BioNTech, see screenshot below, from the redacted August 2021 CHMP report. You can see that by the July 2021 due date this was still not done and to this day no evidence has been seen that it was ever done.

In the same EMA CHMP report, a new obligation was made for BioNTech to fulfill- a request for the same characterization exercise to be done for at least three additional tozinameran batches (modified mRNA, drug substance). As you can see this was not fulfilled by July 2021.

And according to the last updated (Feb 2, 2023) Comirnaty: EPAR report, this obligation is still left unfulfilled.
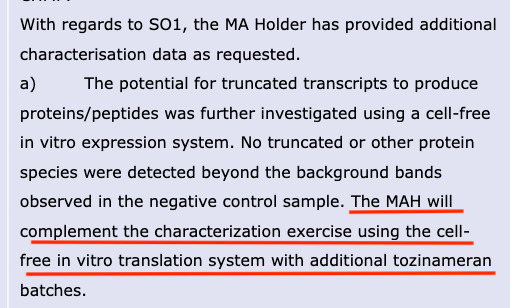
Unbelievably, Cooke references in her letter (see screenshot below) that the “companies developing and marketing mRNA vaccines are conducting planned additional non-clinical studies…to further characterise and assess the biodistribution and degradation of mRNA and the spike protein. Results from these studies will be submitted for assessment by EMA in 2023 and 2024.”

She fails to state that these studies had a due date of July 2021 but were never fulfilled by the “companies.” Her letter reveals that these critical studies have been pushed to 2023 and 2024!
This is evidence that the EMA has no plans to reinforce the specific obligations they made on BioNTech and Pfizer from late 2020.
It’s worth noting, the largest study of its kind from King Fahad University Hospital in Khobar, Saudi Arabia, linked mRNA vaccines to triggering autoimmune diseases, which TrialSite has reported on. ‘Molecular mimicry’ was stated as a concern in the EMA report mentioned previously, is the same hypothesis put forward in the study as the mechanism associated with mRNA vaccines causing an autoimmune process.
In my interview with leading genomics scientist and R & D lead on the Human Genome Project, Kevin McKernan stated, “We have an mRNA product where every single uridine has been replaced with N1 Methylpseudourine, which the body has never seen before. They [BioNTech and Pfizer] picked stop codons that are the most notorious for creating errors. They [were aware of the problem but didn’t properly fix it. This means that when ribosomes go to read the template, they’re really confused as they’ve never seen it before.”
McKernan co-wrote a paper with Dr Peter McCullough and Anthony Kyriakopoulos entitled ‘Differences in Vaccine and SARS-CoV-2 Replication Derived mRNA: Implications for Cell Biology and Future Disease.’ The authors concluded that ‘synonymous codon changes incorporated into mRNA vaccines can alter the expected encoded protein conformation as the translation speed and efficiency can result in different protein folding…Codon optimization strategies for the development of mRNA vaccines can result in immune de-regularities, affect epi-transcriptomic regulation, and can lead to disease progression.‘
Röltgen et al. study published in Cell, revealed that vaccinal modifed mRNA and the “vaccine spike antigen and mRNA persist for weeks in the human lymph nodes.”

Cooke is obliged to mention this study in Cell, thanks to MEP Robert Roos referencing it in “a subsequent letter” to her. She flatly writes, “we can confirm that this study was reviewed by our scientific experts and it does not change the overall benefit-risk assessment..”
Another study by Castruita et al., showed that the SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination.
The fact that Cooke is still pushing the false notion that the mRNA gets degraded quickly, within 6-9 days, given the amount of evidence we have now- makes her guilty of spreading misinformation.
Cooke has the audacity to go on to write “ these animal studies give reasonable confidence that when vaccines are given to humans, no safety problems due to the temporal accumulation of lipid nanoparticles and mRNA in organs are expected.”
I asked pharmacist, Maria Gutschi, PharmD, about these particular animal studies. Gutschi, who has over 30 years of experience in hospital, community, and government, and has independently analyzed the quality issues of Pfizer/BioNTech vaccine identified by the European Medicines Agency, in an informative presentation video.
She informed me, “The safety data performed by Pfizer/BioNTech included two repeat-dose toxicity studies in rats. A variety of mRNA prototypes were studied including those coding for the receptor-binding domain, self-amplifying RNA as well as two versions of BNT162b2 (V8 and V9). Only the whole formulation was used so there is no toxicological data on the LNPs [lipid nanoparticles] alone or for the specific novel excipients. The EMA noted that in repeat-dose toxicity studies in rats, functional hepatic and/or biliary effects were found; these included increased hepatocellular, periportal vacuolations and levels of liver enzymes. Histopathology of the rats sacrificed at Day 17 showed inflammatory signs at the injections site, perineural tissue of the sciatic nerve and surrounding bone. There were also significant hematological responses including very strong increases in neutrophils, eosinophils and basophils and acute phase proteins and a decrease in red blood cell parameters. The spleen showed increased hematopoiesis in half of the animals at Day 17. Edema and erythema at the injection site appeared to increase with each injection and lymph nodes were enlarged, especially with the higher dose study. However, no further experimental toxicological studies were requested by the EMA. Available data were considered sufficient in combination with the clinical trials where the whole formulation (modRNA plus LNP) was to be assessed.”
So, even in the animal studies there were adverse events identified- yet Cooke falsely claims there were “no safety problems.”
Cooke goes on to write, “ In a follow up letter sent on 31 March 2023, Mr Roos also asked me to explain how the COVID-19 vaccines can be considered safe and effective for new-born children, women of childbearing potential, pregnant women and their children, when the effects on fertility have not been reviewed since these groups have been excluded from the clinical trials.”
She responds by referencing the highly flawed and biased observational studies of “65,000 pregnancies…providing necessary assurance about the safety of this vaccine in this population.”
I have written before on the flaws of these observational pregnancy studies and the confounding factors limiting them. For example, from the 10,000 pregnant women who were actually vaccinated from the group of 65,000- only 1.7% were given an mRNA vaccine in their first trimester.
Other important facts that Cooke omits about these studies are: they have not been independently verified; they are also not randomised controlled studies either-which is the gold standard of any trial.
Where is the patient-level data from these 65,000 records?
Has the EMA verified the 65,000 patient records or are they just taking the authors- Lipkind et al.’s word for it?
All of which have ties to the pharmaceutical industry. For example, Heather Lipkind is on the Pfizer COVID-19 Vaccine in Pregnancy Data Safety Monitoring Board. Kimberly Vesco, disclosed she receives institutional support from Pfizer. Candace Fuller disclosed she receives institutional research funding from Pfizer and Johnson & Johnson.
Cooke also fails to mention the alarming data found in the preclinical DART (Developmental and Reproductive Toxicology) study conducted on rats by BioNTech, which showed an increase in miscarriage (preimplantation loss) rate! See screenshot from page 55 of TGA’s nonclinical evaluation report.
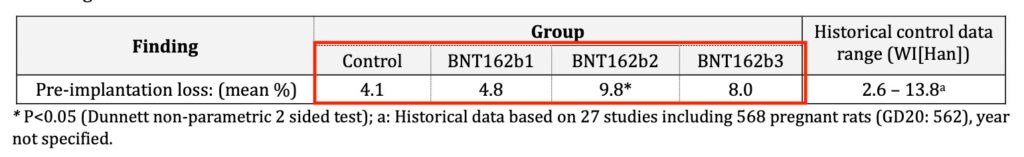
Notice, the more than doubling of the pre-implantation loss rate of the BNT162b2 (the rolled out Pfizer-BioNTech mRNA vaccine) group of 9.8% compared to the control group of 4.1%.
This study was actually criticised in the EMA’s CHMP leaked Rapporteur Rolling Review from November 2020. The assessors wanted BioNTech to justify the choice of the rat “as a relevant animal species (rodent placental antibody transfer during the latter part of gestation is not considered similar to human antibody transfer during the third trimester of gestation.)”

Turning back to the Roos’ question: “how the COVID-19 vaccines can be considered safe and effective for new-born children, women of childbearing potential, pregnant women and their children?”
They are not.
Only recently, I found a damning 8-page document released as part of this month’s court-ordered Pfizer data dump: Pfizer’s Pregnancy and Lactation Cumulative Review (of adverse events) from early 2021. You can read the alarming findings found in my analysis of it here.
It shows evidence of harms to fetuses being exposed to the vaccine transplacentally and harms to infants being exposed to the vaccine via trans-mammary route (through breast milk of vaccinated mothers).
A study by Hanna et al. showed COVID-19 vaccine mRNA in the breast milk.
All this damning data was known by the FDA and other regulators including EMA around the same time (early 2021) just before health authorities started pushing it onto pregnant and lactating women that Spring.
Furthermore, my recent analysis of the EU’s Periodic Safety Update Report (PSUR#1) for Children’s Health Defense Europe, also showed alarming data known from the summer of 2021.
Below is a screenshot revealing an overview of the data from the first 6 months of 2021.

In conclusion, Cooke writes “EMA will continue to closely monitor the safety of COVID-19 vaccines. Any new findings that should emerge in the future will be closely analysed and if confirmed, adequate and immediate action will be taken..”
Really, Ms Cooke? I highly doubt that.
Special thanks to Dr David Wiseman, Maria Gutschi and a scientific expert who prefers to remain anonymous.
Originally published on Sonia Elijah Investigates
Suggest a correction