EMA’s latest bombshell instalment of damning data confirms their failure: PSUR #3, the pregnancy and lactation cases
Thanks to a Freedom of Information Act request, the EU’s Periodic Safety Update Report #3 (PSUR #3), covering the 6-month period of 19 December 2021 through to 18 June 2022, recently became available on the Austrian Politics and Science blog, tkp.
My analysis of PSUR #1 was published in Children’s Health Defense Europe, which covered adverse events reported over the first 6 months of 2021 for the Pfizer-BioNTech mRNA COVID-19 vaccine. At this current time, PSUR #2 and the appendices for PSUR #1 and #3 have not yet been released.
This specific article is part 1 of the analysis of PSUR #3, the third pharmacovigilance document requested by the European Union’s drug regulator, the European Medicines Agency (EMA), prepared by Pfizer/BioNTech and signed off by the Vice President of Worldwide Medical and Safety at Pfizer, Barbara De Bernardi MD, on 18 August 2022.
An overview of the data
- 508,351 case reports (individuals) containing 1,597,673 events
- Three times the number of cases were reported for women than men
- 1/3 of all cases were classified as serious
- 3280 deaths reported
- 60% of cases were reported with either outcome unknown or not recovered
- 92% of cases did not have any comorbidities
- Highest number of cases occurred in the 31-50 year age group
- Germany had the highest recorded number of cases (22.5% of all reported worldwide cases)
This report focuses on the pregnancy and lactation adverse event cases in the 396-page bombshell report.
Breast-feeding infant cases
It is important to note that upon further review of PSUR #1, something extremely disturbing surfaced – adverse events were reported for breast-fed babies, indirectly exposed to the Pfizer-BioNTech mRNA shot, by their vaccinated mothers. The screenshot below is taken from page 165 of PSUR #1.

The fact that 2 cases from the post-marketing (PM) data involved babies who were indirectly exposed to the Pfizer-BioNTech mRNA vaccine (BNT162b2) via the trans-mammary route (through the breast milk) and consequently suffered a stroke (central nervous system haemorrhages and cerebrovascular accidents), is shocking.
Then, on page 149 (screenshot below), 3 more cases of babies suffering from neurological adverse events, for example, convulsions, from being indirectly exposed to the vaccine via their vaccinated mothers’ breast milk, were recorded.

This pattern was also observed in PSUR #3, the screenshot below is taken from page 80.
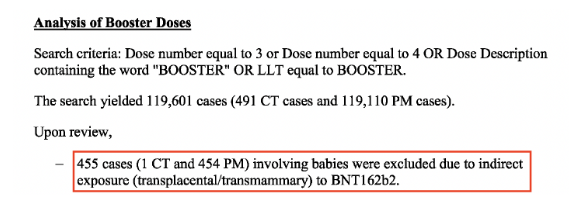
From the analysis of booster doses (> 2 dose primary series), a staggering 455 cases were recorded during the 6-month reporting interval (1 from the clinical trial data and 454 recorded from the post-marketing data) involved babies whose cases “were excluded due to indirect exposure (transplacental/transmammary) to BNT162b2”
A further example, shown in the screenshot below taken from page 239, reports 4 cases (babies) suffering from respiratory adverse events of special interest (AESI), which were “determined to be non-contributory and were not included in the discussion since these cases involved exposures to the vaccine during the mother’s pregnancy or through breastfeeding.”
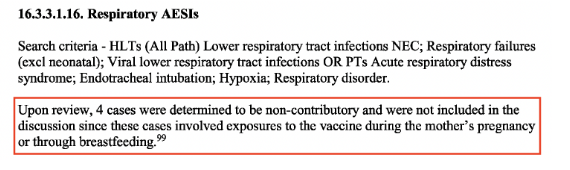
In both PSUR reports the same reason is given by Pfizer/BioNTech for why these cases “are not included in the discussion” because they “were determined to be non-contributory” since they involved babies “who were indirectly exposed to BNT162b2.”
Given the gravity of this important safety signal affecting nursing babies, to brush over the fact that these infants’ adverse event cases were non-contributory because they were indirectly exposed to the vaccine via breast milk- is unconscionable.
Results from a ground-breaking September 2022 study by Hanna et al., published in JAMA Paediatrics, researchers found trace amounts of the COVID-19 vaccine mRNA in the breast milk of lactating women, as soon as one hour after vaccination. They went on to state, “We speculate that, following the vaccine administration, lipid nanoparticles containing the vaccine mRNA are carried to mammary glands via hematogenous and/or lymphatic routes.”
Remember the unwavering yet completely unsubstantiated claim made by drug regulators and health authorities across the globe that the mRNA vaccine just stays in the arm. Well, that couldn’t have been further from the truth.
In the recent leaked letter by the EMA, Executive Director, Emer Cooke, to the Chair of COVID-19 Special Committee, MEP Kathleen Van Brempt, Cooke begrudgingly admitted, “that the lipid nanoparticles can distribute rather non-specifically to several organs such as liver, spleen, heart, kidney, lung and brain, with the liver appearing to be the organ where the lipid nanoparticles distribute most.”
Her admission was made on the heels of the Therapeutics Goods Administrations (TGA) of Australia’s evaluation report on Pfizer’s nonclinical biodistribution study, raised by the MEP, Robert Roos, at the March 27, 2023, Special Committee hearing on COVID-19. The January 2021 TGA report, alarmingly revealed that the lipid nanoparticles which encase the mRNA, travel to the liver, spleen, brain, eyes, bone marrow, adrenal glands, ovaries and testes– nearly every organ tissue.
The pregnancy cases (cumulative clinical trial data)
The pregnancy cases arising from the cumulative clinical trial data in PSUR# 3, originated from Pfizer’s phase 1/2/3 clinical trial through to June 2022. Even though pregnant women were excluded from Pfizer’s pivotal trial, some of the female participants became pregnant. As part of the approval letter for the emergency use of COMIRNATY (marketing name for Pfizer-BioNTech mRNA vaccine), the World Health Organisation (WHO) requested that BioNTech, the marketing authorisation holder, monitor their outcomes.
There were 697 pregnancy cumulative cases reported, which comprised of 597 mother cases and 100 baby/foetal cases. ‘431 cases reported exposure to vaccine in utero without the occurrence of any clinical event’ in the mother cases. The following is a breakdown of the 166 mother cases which did report adverse clinical events. The numbers in brackets reflect the number of frequently reported events.
- ~ 1/5 of all mother cases reported serious adverse events (139)
- spontaneous abortions (46)
- Pre-eclampsia (7)
- Cephalo-pelvic disproportion (6)
- Abortion missed, Foetal death, postpartum haemorrhage, premature separation of placenta (4 each)
- Abortion threatened, ectopic pregnancy, gestational hypertension, premature delivery, premature labour (3 each)
- Abortion incomplete, hyperemesis gravidarum, maternal exposure via partner during pregnancy, miscarriage of partner, uterine disorder (2 each)
- COVID-19 (9)
- Anaemia (2)
From the list above, it’s note-worthy to point out that “maternal exposure via partner during pregnancy” and “miscarriage of partner” refers to cases of women being indirectly exposed to BNT162b2 by their vaccinated partners. This importantly relates to vaccine shedding, which was on Pfizer’s radar even before its clinical trial commenced but was later widely reported as a myth to the public. According to Pfizer’s own clinical trial protocol, cases of pregnant women who were indirectly exposed to the vaccine by their partners (who participated in the trial) were classified as ‘Exposure During Pregnancy’ and immediately reported to Pfizer Safety on the Vaccine Serious Adverse Event Form within 24 hours of the investigator’s awareness. The pregnancy was to be followed up by the investigator with Pfizer Safety being notified of the outcome.
The baby/foetal cases (cumulative clinical trial data)
What’s disturbing is that a staggering 98 out of the 100 baby/foetal cases were reported as serious. The screenshots below reflect their appalling outcomes.

For the 68 baby/foetal cases showing ‘live birth without congenital anomaly,’ the serious adverse event outcomes can be read in the screenshot below.

There was also one case of still birth without foetal defect during the reporting period, which was coded as Neonatal respiratory distress syndrome.
The pregnancy cases (from the post-authorisation data)
According to the post-authorization data, there were 3642 pregnancy cases with 1898 cases pregnancy outcomes provided during the reporting period (Dec 2021-June 2022). Germany had the highest number of pregnancy cases (837) in the reporting period, followed by the UK with 475 cases. The table of those 1898 outcomes can be seen below.
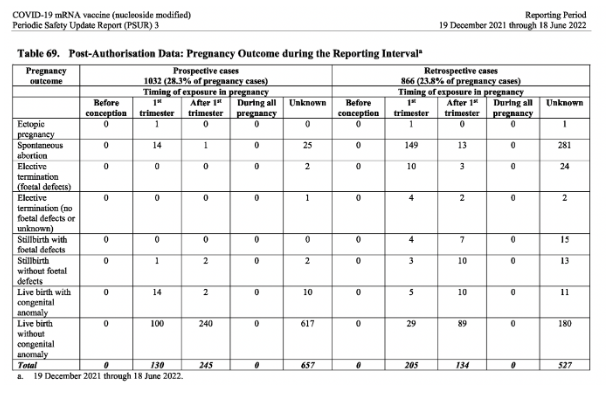
Significantly, a third of the pregnancy outcomes provided during the reporting period were negative.
Spontaneous abortion: 483
Live birth with congenital abnormalities: 52
Still birth with foetal defects: 26
Elective termination (because of foetal defects): 39
Out of the 3642 pregnancy cases, 322 were classified as baby/foetal cases and 3320 were mother cases.
The baby/foetal cases (post-authorisation data)
90% of the 322 baby/foetal cases were classified as serious. There were 39 cases of ‘live birth with congenital anomaly.’ The screenshots below, show the frightening range of those defects.

There were 37 cases of spontaneous abortion in the baby cases with reported events of ‘Foetal growth restriction (18) congenital anomaly (8), Foetal heart rate abnormal (3), Cytogenetic abnormality Foetal vascular malperfusion (2 each).’
In 4 cases the mother had an underlying medical history but for the remaining 33 cases, the report states, ‘there was limited information regarding obstetric history or co-suspect medications of the mother, which precluded meaningful causality assessment.’
There were 23 cases of reported elective termination of pregnancy. 22 out of the 23 cases ‘reported elective termination due to foetal defects.’ There were a further 21 cases of still births, with just over 70% of those cases reporting foetal defects.
In stark contrast to the damning data, the report concludes: ‘There were no safety signals regarding use in pregnant/lactation women that emerged from the review of these cases..’
Furthermore, throughout the ‘Use in Pregnant/Lactating Women’ section in PSUR #3, the following dismissive and recurring statement is made, ““There was limited information regarding mother’s obstetric history, which precluded meaningful assessment.”
My recent investigative report on Pfizer’s Pregnancy and Lactation Review, just released in April per court-order by the FDA, 2 years after it was signed off, contained similar damning adverse events, such a spontaneous abortion and preterm delivery of foetuses, exposed to the vaccine trans-placentally or trans-mammary (through the breast milk) after their mothers were vaccinated. Adverse events such as facial paralysis and lymphadenopathy were also reported in infants, indirectly exposed through the breast milk of their vaccinated mothers.
Earlier this year, a paper was published in the Journal of American Physicians and Surgeons by Thorp et al., which revealed an astonishing volume of global adverse event counts for COVID-19 vaccines reported over 18 months, compared to 282 months for influenza vaccines, (see screenshot of Table 1 below).

Given the abundance of damning evidence, it has become increasingly apparent that serious negative pregnancy outcomes observed post-vaccination can be seen as a contributing factor to the decline in the birth/fertility rate, reported in countries, which rolled out the mRNA vaccines.
In November 2022, shocking results were revealed in a working paper by the Federal Institute for Population Research, entitled, “Fertility declines near the end of the COVID-19 pandemic: Evidence of the 2022 birth declines in Germany and Sweden”.
The paper stated, “The seasonally adjusted monthly TFR of Germany dropped from 1.5-1.6 in 2021 to 1.3-1.4 in 2022, a decline of about 14 %. In Sweden, the corresponding TFR dropped from about 1.7 in 2021 to 1.5-1.6 in 2022, a decline of almost 10 %. There is no association of the fertility trends with changes in unemployment, infection rates, or COVID-19 deaths. However, there is a strong association between the onset of vaccination programmes and the fertility decline nine months after of this onset.”
What is noteworthy is that even in the EMA’s Risk Management Plan version 9.0, revised in November 2022 for COMIRNATY (both monovalent and bivalent versions), it states on page 111: “The safety profile of the vaccine is not fully known in pregnant or breastfeeding women due to their initial exclusion from the pivotal clinical study however, post-marketing experience in pregnant women is available.
So, we have on record that the EMA’s current position is that the safety profile of the vaccine is not fully known in pregnant and breastfeeding women.
Secondly, we know the EMA were fully aware of the alarming safety signals found in these Periodic Safety Update Reports (including clinical trial and post-marketing cases), first documented in PSUR #1 because Pfizer and BioNTech compiled these pharmacovigilance documents for the regulator. Yet, the agency shockingly went on to rubber stamp these experimental mRNA products as “safe and effective” for pregnant and lactating women and continue to this day to promote its use for this cohort.
All this culminates to a preponderance of evidence showing that the agency is not only culpable of failing to protect this generation of European citizens but also the next.
Suggest a correction