Is Breastfeeding Post mRNA vaccination Safe?
Pfizer’s own safety reports show otherwise
From the start of the big push to get ‘shots in the arms’ of everyone: the elderly down to 6-month-old infants, including pregnant women- every politician, public official, health authority and regulator emphasized in parrot-like fashion, how the COVID-19 mRNA vaccine stays in the arm and quickly degrades. This was simply not true.
The lipid nanoparticles encapsulating the synthetic vaccinal mRNA travel all over the body- including the mammary glands where they are secreted in the breast milk of lactating women.
This was first demonstrated in Low et al. study, published in August 2021, then the Yeo et al. study, published in January 2022. Followed by Hanna et al.’s September 2022 study: Detection of Messenger RNA COVID-19 Vaccines in Human Breast Milk.
Where the researchers found both Moderna [mRNA-1273] and Pfizer-BioNTech’s [BNT162b2] mRNA present in the breast milk samples.
‘Of 11 lactating individuals enrolled, trace amounts of BNT162b2 and mRNA-1273 COVID-19 mRNA vaccines were detected in 7 samples from 5 different participants at various times up to 45 hours postvaccination.’
Now a new study by Hanna et al., recently published on September 19, 2023, demonstrated similar results for both the Moderna and the Pfizer-BioNTech mRNA vaccines.
‘Of 13 lactating women receiving the vaccine (20 exposures), trace mRNA amounts were detected in 10 exposures up to 45 h post-vaccination.’
The authors went on to state:
Our findings demonstrate that the COVID-19 vaccine mRNA is not confined to the injection site but spreads systemically and is packaged into BM [breast milk] EVs [extracellular vesicles].
From the arm to the breast milk
The graphic below details the authors’ ‘Proposed model of biodistribution of vaccine mRNA to breast milk (BM).’
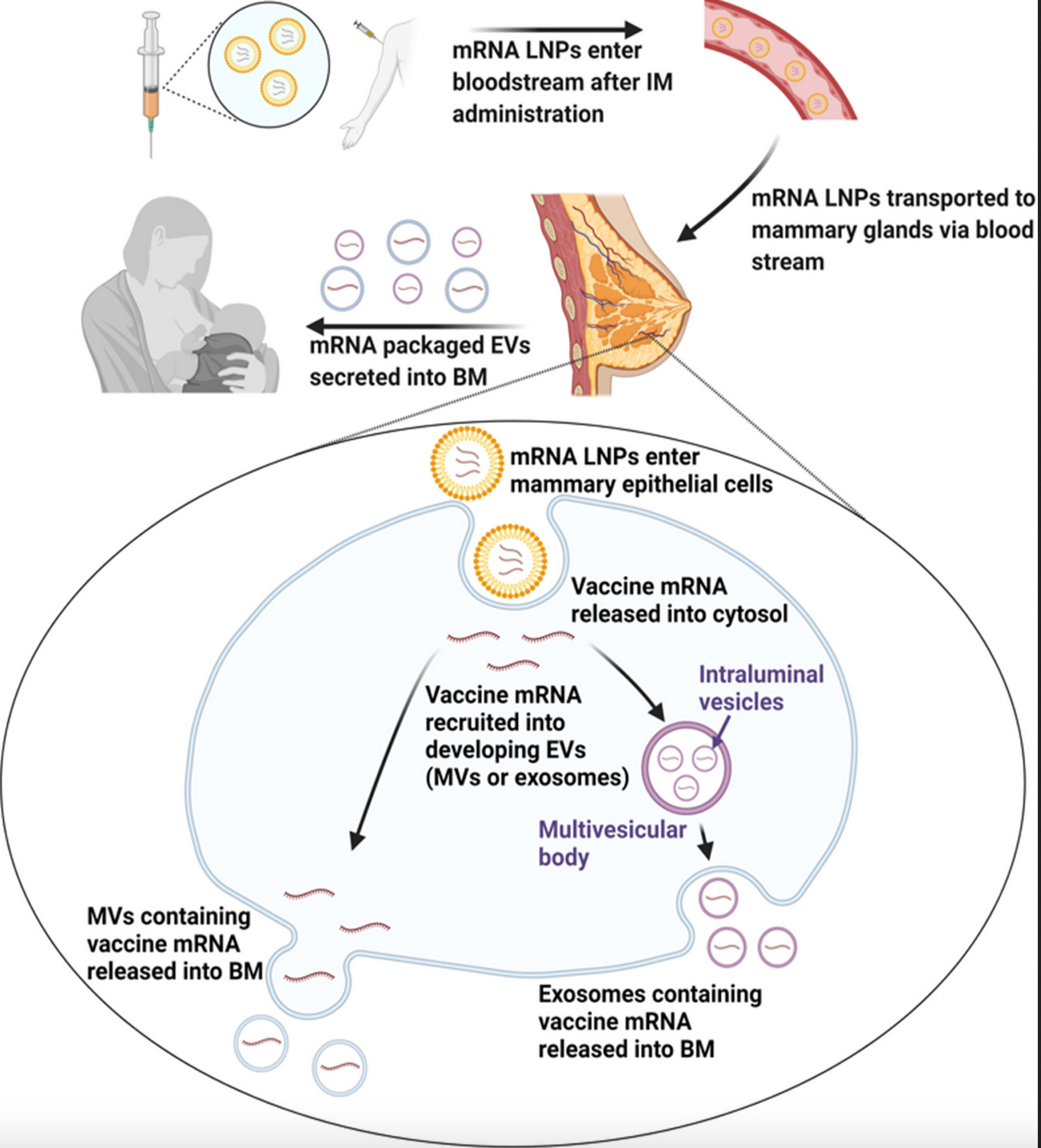
Source: Hanna et al. September 2023 study
What was known from January 2021
Evidence that the Pfizer-BioNTech lipid nanoparticle encapsulated modified mRNA travels all over the body and accumulates in nearly every organ tissue- was known as early as January 2021.
This was revealed in the nonclinical evaluation report of BNT162b conducted by the Therapeutics Goods Administration of Australia (TGA) but despite that knowledge, the regulator went on to provisionally approve the Pfizer-BioNTech COVID-19 vaccine for use in Australia by the end of that month.
It is important to highlight that the manufacturers (BioNTech and Pfizer) never did (nor intended to do) a biodistribution study for the actual modified mRNA encoding for the SARS-CoV-2 spike protein. Somehow, no objections were raised by the regulators because of this nor by the fact that critically important safety studies were never done. The single biodistribution study that was done- Study 185350 on Wistar Han rats, was for the distribution of lipid nanoparticles (containing ALC-0315 and ALC-0159) mRNA encoding for luciferase.
The highlighted sections in the table below, show where the lipid concentration accumulated the most following a single dose injection: the ovaries, bone marrow, liver and adrenal glands.
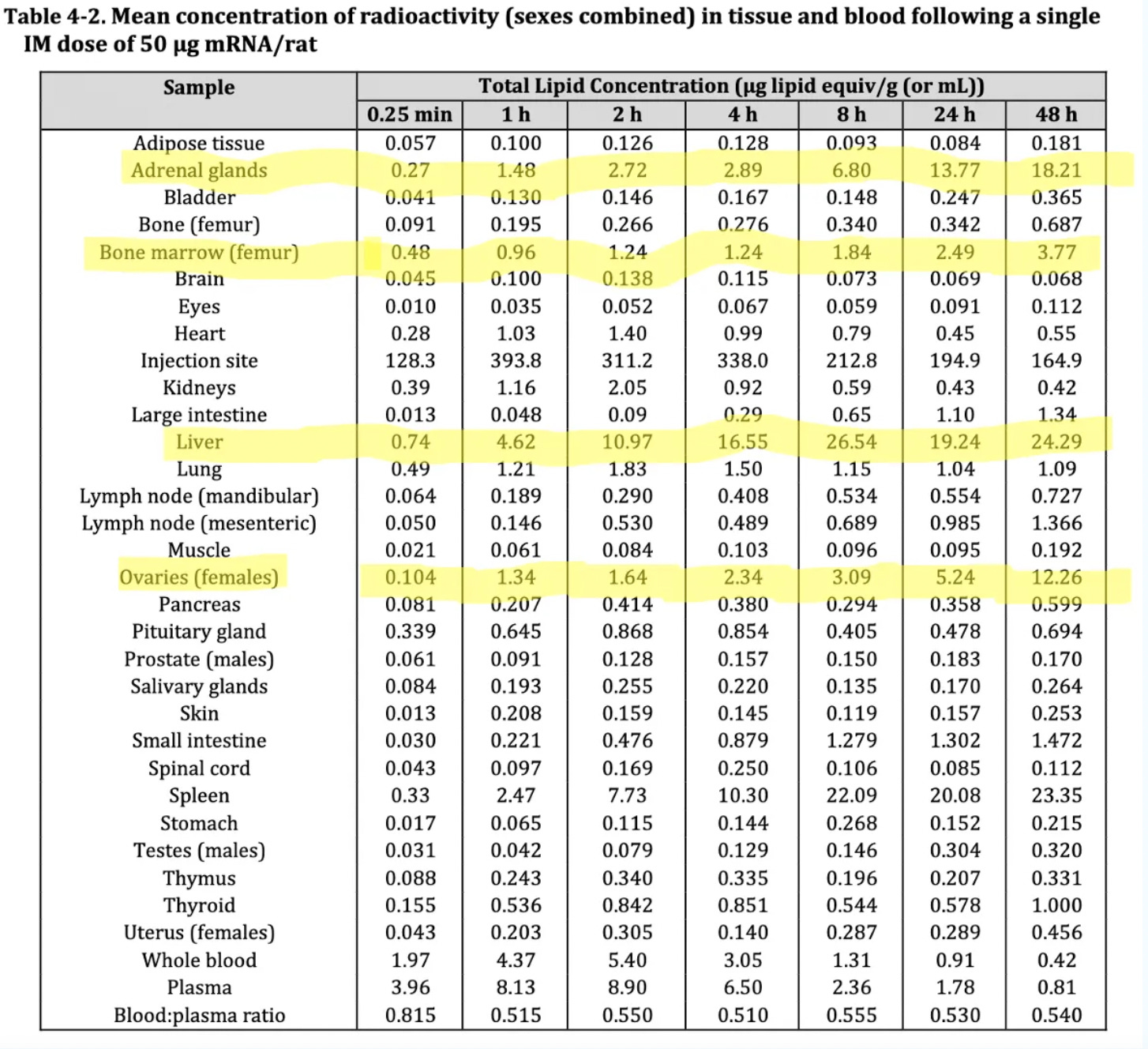
Source: TGA’s nonclinical evaluation report for Comirnaty, BNT162b2 [mRNA] COVID-19 vaccine
It is noteworthy that just like the MHRA’s public assessment report, the TGA report also stated: “It is unknown whether BNT162b2 is excreted in human milk.”
Furthermore, in the MHRA’s original Public Health Assessment report of Pfizer-BioNTech mRNA vaccine on page 21, they write: “In the context of supply under Regulation 174, it is considered that sufficient reassurance of safe use of the vaccine in pregnant women cannot be provided at the present time.”
With the report going on to categorically state: “Women who are breastfeeding should also not be vaccinated.”
However, this message below was slapped on at a much later date.

Somehow, this did not stop health authorities around the world encouraging the mRNA vaccination for pregnant and lactating women from the spring of 2021, despite the regulators stating otherwise.
What is interesting is that following my September 4, 2022 report on the contradictory advice between the regulators and health authorities, which was originally published on Trial Site News, the MHRA made the following announcement on the UK Government website, on September 5.
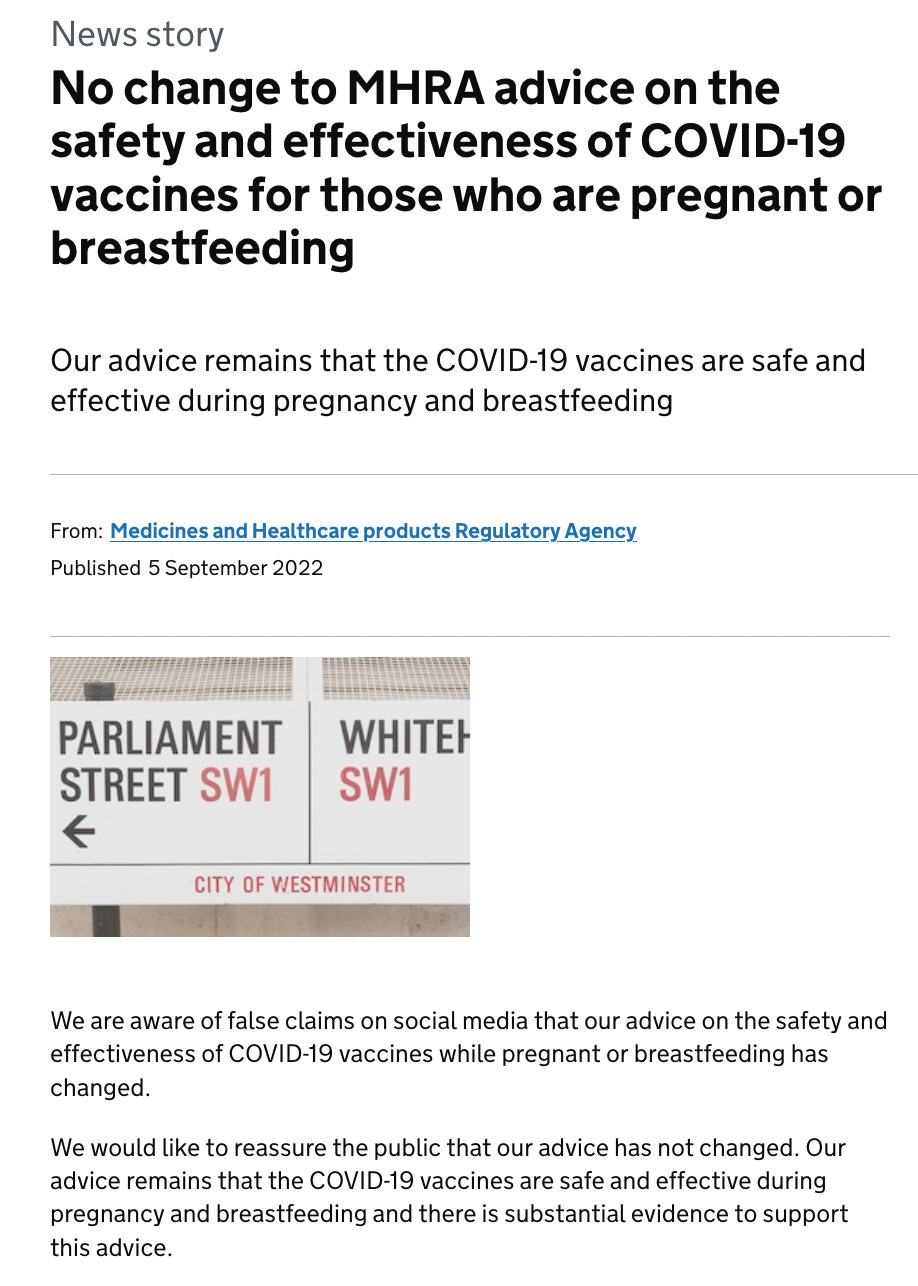
Problems with the authors’ belief
Turning to the recent Hanna et al. study, the authors’ state their belief: “breastfeeding post-vaccination is safe,” which appears to be formed on the basis that:
only trace quantities are present and a clear translational activity is absent..
However, an automated Western Blot system (instead of a traditional one done by hand) was used to demonstrate that ‘translational activity is absent.’ Western Blotting is a method used to identify specific proteins. In the Breast milk EVs (shown by lanes 4-7) no spike protein was detected, meaning the vaccinal mRNA in the breast milk samples did not translate into the spike protein- leading to the authors’ belief that it was safe.
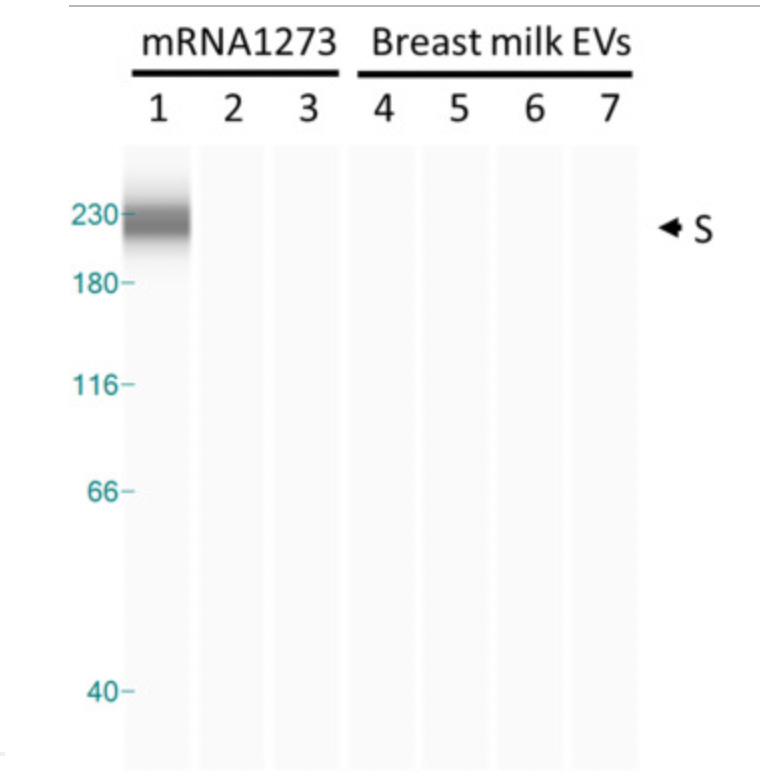
Automated capillary western blot (WES)
This raises concerns, given the findings of my in-depth investigation on the #Blotgate scandal with regards to Pfizer/BioNTech’s own automated Western Blots (which they had the audacity to pass off as traditional/authentic Westerns), which included damning evidence of the data being ‘copy and pasted’- ie. fraudulent.
The failed positive control
‘As a positive control, HT-29 cells were treated with mRNA-1273 [Moderna mRNA vaccine] at various dilutions (1:104, 1:106, and 1:107).’
Lanes 1-3 in the image above, depict the positive control, yet lanes 2-3 show no presence of the spike protein, which is unexpected. The authors make note of this fact: ‘However, positive control samples used in concentrations similar to those of BM EVs also failed to induce S protein expression.’ It is unusual that the vaccinal spike protein was absent in the positive control lanes (2-3) which does not instil confidence in the validity of the test.
The many limitations of the study
The authors go on to disclose the limitations of their study:
Other limitations include the potential underestimation of the mRNA concentrations due to differences in the mothers’ collection techniques and storage conditions following self-collection, which may contribute to mRNA degradation. Another limitation includes the limited volume of BM provided by mothers, which limited the feasibility of further experiments. Also, we did not test the possible cumulative vaccine mRNA exposure following frequent breastfeeding in infants, which can add up to 150–200 mL/kg/day of BM.
It is highly concerning that the study did not test the possible cumulative vaccine mRNA exposure in infants, considering how frequent young babies are breastfed.
The need for further investigation
The authors make a point of stating:
Although the vaccine mRNA appears to be translationally inactive, further investigation is required to determine the minimum amount of mRNA needed to elicit an immune response in newborns.
Nevertheless, since the minimum mRNA vaccine dose to elicit an immune reaction in infants <6 months is unknown, a dialogue between a breastfeeding mother and her healthcare provider should address the benefit/risk considerations of breastfeeding in the first two days after maternal vaccination.
The possible passage of the vaccine mRNA to breast milk (BM), resulting in neonatal exposure, was not investigated. Notably, the Centers for Disease Control and Prevention (CDC) does not recommend COVID-19 vaccination in infants <6 months of age because of the lack of safety studies and the possible interaction with other routine vaccinations in this age group.
The devastating harms to breast-fed infants from Pfizer/BioNTech’s own data!
This brings me to the disturbing discoveries found after analysing Pfizer’s Periodic Safety Update Reports for the European Medicines Agency (EMA), starting from January 2021. This report series has been published in Children’s Health Defense, Europe.
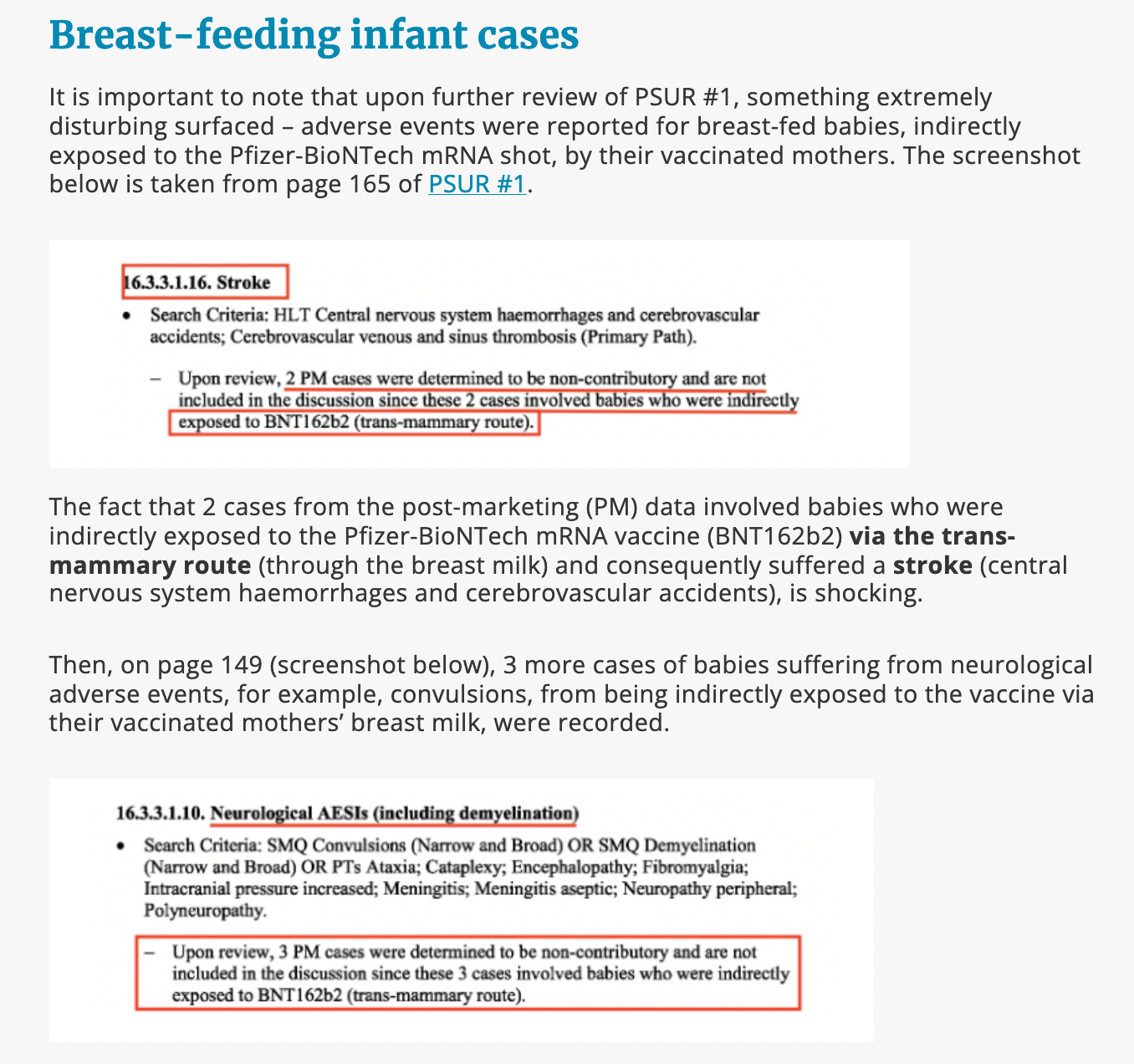
Extract from: EMA’s latest bombshell instalment of damning data confirms their failure: PSUR #3, the pregnancy and lactation cases, report
It is deeply shocking that Pfizer did not even include the breast-fed infant cases to the “discussion” as they were deemed to be “non-contributory” due to indirect exposure “to BNT162B2 (trans-mammary route)” and the regulator, the EMA, approved that decision.
In addition, Pfizer’s Pregnancy & Lactation Cumulative Review document, revealed even more damning cases of harms to breast-feeding infants exposed to the vaccine via the breast milk.
I was one of the first journalists to discover this document from the monthly data dump of Pfizer papers. My report was published in Trial Site News, on April 22, 2023. The screenshot below is taken from my original report.

Source: Pfizer’s Pregnancy & Lactation Cumulative Review Reveals Damning Data in Trial Site News
You can watch me discuss these findings with Clayton Morris on Redacted.
Originally published by Sonia Elijah investigates, September 23, 2023
Suggest a correction








