RSV: Fact and Fiction With Dr. Meryl Nass and Brian Hooker, Ph.D.
On a recent “Doctors & Scientists” episode on CHD.TV, Children’s Health Defense’s Brian Hooker, Ph.D., and Dr. Meryl Nass discussed the science related to respiratory syncytial virus (RSV) and Big Pharma’s new RSV immunization agenda.
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website.
Respiratory syncytial virus, or RSV, has been dominating the news since the recent approvals by the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) of new RSV vaccines for infants, pregnant women and the elderly.
The new “therapies … are now sort of a boon” for Big Pharma, according to Dr. Meryl Nass, internist and member of the Children’s Health Defense (CHD) scientific advisory committee.
On a recent “Doctors & Scientists” episode on CHD.TV, Brian Hooker, Ph.D., CHD’s senior director of science and research, hosted Nass, who shared a presentation on the science behind RSV and Big Pharma’s new RSV immunization agenda.
Nass led the conversation, showing slides to highlight her main points. This article will summarize her presentation about RSV without attribution, except for comments and direct quotations from the two speakers, and to cite sources.
How dangerous is RSV?
RSV is a common respiratory virus that usually causes mild cold-like symptoms but in rare cases can lead to hospitalization and death in infants and the elderly.
The number of people who contract RSV is unknown because the virus is rarely diagnosed unless one comes to a hospital and is tested.
In very young infants, RSV can cause difficulty breathing, which may call for hospitalization, IV fluids and extra oxygen due to the thick fluid in the lungs.
“It turns out that virtually every baby, even if they’re hospitalized, will get over this with no chronic problems,” Nass said.
By the age of 2, 97% of all babies have been infected with the RSV virus, which confers partial immunity, making any subsequent episodes less severe.
According to a 2021 study from the CDC reviewing 12 years of data, out of approximately 4 million babies born in the U.S. each year, for infants under age 1 — the age group under 50 that carries the highest burden of death from RSV — 26 death certificates list RSV, and only 17 actually blame RSV as the underlying cause of death.
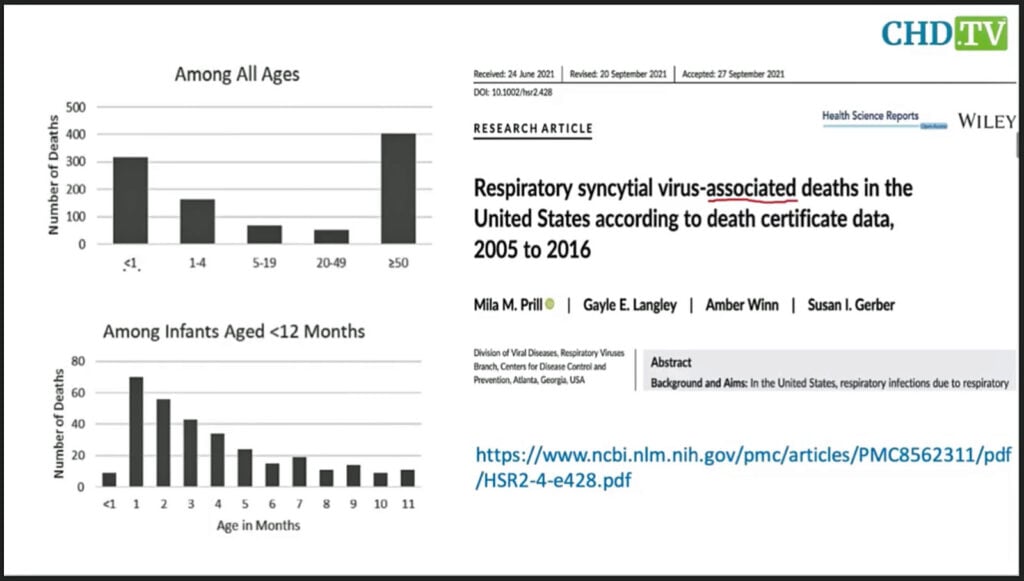
In 12 years there were 1,001 death certificates in all age groups in the entire U.S. that listed RSV. That represents fewer than 100 people per year.
Nass said the CDC’s graphs could be misleading because they represent 12 years of data, not one year.
“Now, why might CDC want to do that?” Nass asked. “Possibly because they knew that products to prevent RSV were in the pipeline and they wanted to help build a market for them.”
AstraZeneca’s new monoclonal antibody treatment for infants
Before 2023, there was one product to treat RSV, palivizumab, which costs thousands of dollars per dose and was rarely used.
New treatments for RSV include monoclonal antibodies given to newborns to prevent infection, and new vaccines for pregnant women, elderly adults and children.

Normally, a vaccine — or a natural infection — will cause our bodies to make antibodies, but the production of those could take two to four weeks to peak.
For someone with a life-threatening illness or who may be immunocompromised, monoclonal antibodies provide immediate immune system resistance to viruses.
For example, there are monoclonal antibodies for herpes zoster (shingles) and hepatitis B (liver inflammation) viruses, and for the treatment of some cancers and autoimmune diseases.
Some monoclonal products are made from hamster ovaries, which give the products a longer half-life. Monoclonal treatments can have significant side effects.
AstraZeneca’s new product Beyfortus, approved by the FDA in July, is a new monoclonal antibody product designed to be given to infants on the very first day of life and can be given to babies up to 8 months old.
The product has resulted in anaphylactic reactions and rashes in some babies, according to Nass, who expressed concern about giving monoclonal antibodies to newborns, which has not routinely been done before, “and certainly not on the first day of life,” she said.
In its deliberations over the drug, the CDC’s Advisory Committee on Immunization Practices (ACIP) was primarily concerned about whether insurance would cover the drug because it retails for $495 per dose.
“The hospitals do not want to front this money,” Nass said, “unless they know they’re going to be reimbursed. … The CDC has not been able to pressure the insurance companies enough, I guess, to start adding this product … to their insured list.”
Under current law, it can take up to 22 months for that to happen, a process complicated by the fact that Beyfortus is not a vaccine or a drug, but a biologic.
“And so CDC has to do some fancy footwork to, in some cases, call it a vaccine, and in other cases call it a drug in order to have it go through normal regulatory processes,” Nass said.
If a baby only received this treatment on day one and had side effects, the doctor or parent would have to report it to the FDA Adverse Event Reporting System (FAERS), and not to the Vaccine Adverse Event Reporting System (VAERS), which is co-managed by the CDC and FDA.
If, however, the baby received a shot like hepatitis B on the first day along with Beyfortus, it would be reported to VAERS, said Nass. Hooker commented that the confusion would likely result in even more underreporting than such systems currently experience.
“It’s a crazy experiment,” said Nass. “There really isn’t good evidence that it’s saving any lives.”
“There should be some kind of analysis to show people the net gain from using the treatment versus not using it,” Hooker said, adding, “But it seems like this type of analysis is absent.”
Nass pointed out that for the infant RSV vaccine trial, 20 babies died in the placebo group and eight died in the group that received the monoclonal antibodies, but that during the ACIP meeting, the briefer only said there was “a disparity in the deaths.”
She said this disparity “is a hint that somebody was throwing the sick babies into the placebo group” because “it would be extremely unusual, like maybe one in a million … to get this difference in deaths on the basis of chance.”
“So there was probably something wrong with the clinical trial and nobody wants to talk about that,” she said.
Pfizer’s new RSV vaccine for pregnant women
Pfizer has created an RSV vaccine for pregnant women called Abrysvo that claims to produce antibodies to RSV in newborns and provide up to six months of protection.
The FDA approved the drug in August, despite the concerns of some members of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) over the increase in preterm births in the clinical trial.
GSK (formerly GlaxoSmithKline) had been working on a similar RSV vaccine but halted its development last year when its trial revealed the same problem with premature births.
“I worry that if preterm births are in any way a consequence of this vaccine, that would be tragic in many ways,” Dr. Paul Offit said at the May VRBPAC meeting, where he voted against Abrysvo for pregnant women.
“What is the risk/benefit balance when you’re talking about 17 babies that die of RSV a year versus thousands more which will have a preterm delivery?” Nass asked, adding, “We know the babies are going to have more complications of other kinds as a result.”
Hooker expressed concern over pregnant women receiving this vaccine on top of a flu vaccine, the Tdap vaccine and possibly one or more COVID-19 vaccines.
Nass noted that the vaccine will be given during the last trimester of pregnancy.
“This will be the fourth shot in pregnancy. None of them have been proven to be safe. If you look at the labels, none of them claim safety and pregnancy,” Nass said while admitting she had not yet reviewed the label for Abrysvo.
RSV vaccines also available for the elderly
Abrysvo was approved by the FDA in May to protect the elderly from RSV. A similar vaccine called Arexvy was also approved in May.
The CDC claims these vaccines, targeted for adults ages 60 and older, will provide protection from RSV “for at least two winter seasons.”
“It might be 60% effective,” Nass said. “We’re not really sure because the efficacy in the real world is always lower than in clinical trials.”
The CDC claims thousands of elders die every year from RSV, “but it’s never been measured,” Nass said, adding “[These are] probably people who … have COPD [and] are very debilitated … very elderly.”
“Something’s going to knock them over to kill them in the end and it’s often a flu and it could be an RSV,” said Nass, “but were they going to die anyway within a few months, the next time they got a cold or a flu.”
The RSV vaccine contains an adjuvant called QS21, developed from Quillaja saponaria, the soap bark tree. QS21, also used in COVID-19 vaccines, according to Nass, is known to induce inflammatory cytokines.
“We don’t know much about the side effects of these two,” she said.
The RSV vaccine for the elderly carries a $300 wholesale price tag,” Nass said, adding “They certainly may be looking towards a triple whammy in the future of flu, RSV and COVID [shots] yearly.”
Big Pharma — and NIAID scientists — looking to cash in
There has been a very small market for RSV products because they generally only cause a cold, according to Nass. “But the analytics companies are saying that the RSV product market should be $9 billion by the end of this decade,” she said.
“Why did all of these products appear suddenly?” Nass asked.
Hooker answered:
“Well, we have a pipeline now with coronavirus and we also have sort of a void. There was a huge outlay of money because of the wild furor in order to get COVID vaccination. So, you know, now we have to introduce a new product in order to continue to boost sales for companies like Pfizer and Moderna.”
Nass agreed, and said there was another wrinkle: that Dr. Anthony Fauci and “his people” at the Vaccine Research Center at the National Institute of Allergy and Infectious Disease (NIAID) invented an antigen for RSV vaccines and had licensed it to all of these companies.
Nass said:
“And so NIAID and HHS [U.S. Department of Health and Human Services] are going to be getting royalties for these products. And every scientist’s name is on the patent can collect up to $150,000 per year on top of their salary for this product. And then HHS will keep the rest.
“To put that into perspective, Moderna, which has made tens of billions of dollars selling COVID vaccines to the government, has had to pay the NIH $400 million in royalties for the COVID vaccine product it licensed from NIAID.”
Robert F. Kennedy Jr., CHD’s chairman on leave, Nass said, pointed out a couple of years ago that Fauci had been involved with almost every new drug and vaccine coming out in the last number of years.
“I thought it was a remarkable statement,” Nass said. “I didn’t know if I could believe it.”
“And so what you start to see is that the CDC, the NIH, the FDA, they’re all in cahoots,” she said, adding, “NIAID created the product. So FDA gives it a license and CDC markets it. And they all work together.”
“And Fauci helped choose the leaders, the director of the CDC and the commissioner of the FDA,” she said.
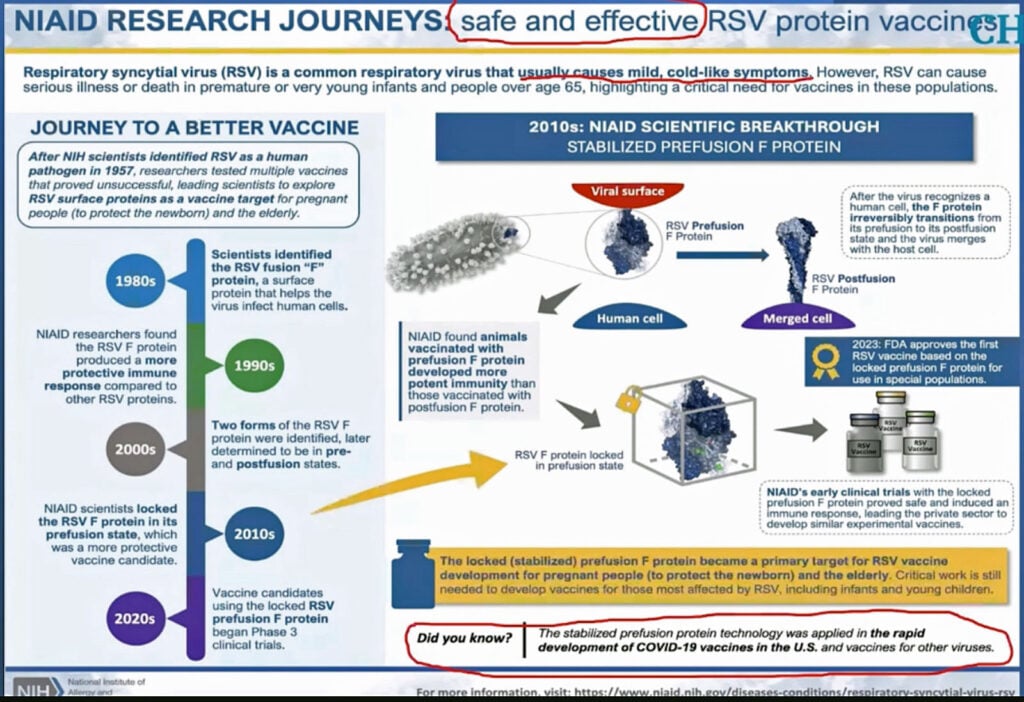
Nass showed a patent for “Prefusion rsv f proteins” which listed among its inventors Barney Graham, formerly the deputy director of the Vaccine Research Center at NIAID, and now the senior advisor for Global Health Equity at Morehouse School of Medicine in Atlanta.
She showed an image from Nature magazine of an advertisement by NIAID for the same product. “Maybe that’s why Nature has run these phony articles on COVID and other things,” she said, adding:
“They’re looking for companies to pay them money for their prefusion f protein [RSV antigen]. And the companies know if they do this, that Fauci and his minions at the NIH are going to make it very easy to get their products licensed. And the more it costs, the more royalties they can pay.”
Nass next showed an article by Fauci titled, “Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses,” where he says that current respiratory vaccines are not very effective and that we should be “rethinking, from the ground up, all of our past assumptions and approaches to preventing important respiratory viral diseases and working to find bold new paths forward.”
Hooker asked, “Is this a moment of clarity, or is it just a ruse to sell better vaccines down the line?”
“It’s throwing more and more resources into the vaccine program,” Nass said.
Nass showed a CDC press release from August advertising the infant RSV vaccine, calling the drug “a powerful new tool to protect infants from the leading cause of hospitalization.”
“Every newborn will get this,” Hooker said.
“That’s their plan,” Nass said, adding, “We recommend that that does not happen.”
“There are already RSV viral strains that are resistant to this monoclonal,” she said. “We can assume that they will increase and soon this product will be of no use whatsoever.”
Regarding the monoclonal antibodies used for COVID-19, Nass pointed out that they were all approved under emergency use authorization. “It’s unclear how well they worked,” she said. “Don’t allow the government to experiment with your newborn.”
Fast-track drug approval reveals problems with our regulatory structure
Nass showed an article from the British Medical Journal revealing the percentage of public health agency budgets derived from pharmaceutical companies.
“The extraordinary answer is that for Australia, Europe, U.K., and Japan, 85% or more of their funding comes from the pharmaceutical companies they regulate,” Nass said, adding, “That’s a very scary thought. In the United States, it’s 65%.”
The cost for a drug to be evaluated by the FDA is about $4 million, according to Nass. “So a company pays FDA $4 million, and they get almost a guarantee — more than two-thirds likely — that their drug will be approved.”
Nass said that 68% of the drugs going through the FDA are now on a fast-track designation. She added:
“The United States Congress has seen fit to create four fast-track pathways by which drugs can be approved even quicker than usual.
“And companies have to pay more to get an approval for FDA to give them a fast-track designation. If they get that designation, most of them indicate FDA has to give them an answer, a yes or no on licensure, within six months.
“It used to be on average about seven years. Now, six months — whether or not FDA has had time to read all the documentation and review it. They’ve got to give a thumbs up or thumbs down. That’s a big problem.”
The FDA is approving 14 new drugs each month, up 20% year-over-year, according to BioWorld.
“What a surprise,” Nass said.
Nass discussed the Vaccine Injury Compensation Program (VICP) and the waiver of liability it gave to vaccine manufacturers for childhood vaccines.

She also discussed a 2016 bill called the 21st Century Cures Act, which included a provision that vaccines the CDC recommends for pregnant women would go into the childhood injury bucket “so that manufacturers would no longer have any liability for injuries,” Nass said.
“So as long as you [the manufacturer] could get your vaccine added for pregnant women — like a COVID vaccine, for example, or this RSV vaccine — there would be no liability,” she said. “You would just have this 75 cents per dose excise tax.”
Women injured by these vaccines would need to apply to the VICP, Nass said, “and if you are lucky, you might get something.”
“The passage of this bill in December of 2016 opened up a gold rush for vaccine manufacturers and pregnant women were the target,” she said. “And so you’re going to be seeing more and more vaccines for pregnancy rolled out.”
Nass shared a favorite quote by Daniel Horowitz:
“Warp speed was not an anomaly. It’s a new framework that requires bold new leadership to reset the great reset on bodily autonomy and informed consent.”
Nass said:
“That means they have rolled over bodily autonomy and informed consent, even though there are laws enshrining these principles in the United States. And we need to get them back. And this needs to be really one of our major focuses as we move forward.”
This article was originally published by The Defender .
Suggest a correction