CDC and FDA contracts show they prepared for massive amounts of serious adverse events months before Covid-19 vaccines were rolled out
A 142 page freedom of information request reveals shocking Covid-19 vaccine adverse event contract documents between the CDC, FDA and General Dynamics Information Technology dated August 27, 2020, four months BEFORE the Covid-19 vaccines were introduced to the general population.

As you can see the contract is dated August 27, 2020, the Covid-19 vaccines began rolling out to the population in the middle of December 2020.
The contract shows the CDC and FDA are seeking assistance from General Dynamics Information Technology to provide support related to the Vaccine Adverse Event Reporting System (VAERS) for Covid-19 vaccines.
What is extremely alarming is the “description of work” section of the the contract. It says, “The contractor shall implement a staffing and operations plan focusing only on vaccine adverse event (VAE) reports after SARS-CoV-2 vaccines. The total number of reports received during periods of peak activity (which are not expected to reflect sustained activity) is expected to be 1,000 reports per day, with up to 40% of the reports serious in nature.”

So, to recap, the CDC and FDA prepared a contract 4 months before the vaccine roll-out and expected 1,000 adverse events per day (7,000 per week), 40% of which were estimated to be serious.
In March 2021, about three and a half months after the Covid-19 vaccines were introduced to the public, the CDC and FDA amended the contract with General Dynamics Information Technology. The amendment was seeking 6 months of assistance in dealing with a backlog of an estimated 115,000 adverse event reports.

Four days later the contract is amended again asking General Dynamics Information Technology to now help in dealing with a minimum 25,000 adverse event reports per week for the next 6 months.

Another amended contract several months later, on September 24, 2021, between the CDC, FDA, and General Dynamics Information Technology shows the CDC and FDA are now asking the company to process a minimum 13,000 extra adverse event reports per week over the next six months.

They amended the contract again in February 2022, requesting General Dynamics Information Technology to continue assisting with a minimum 13,000 adverse event reports per week for another six months.
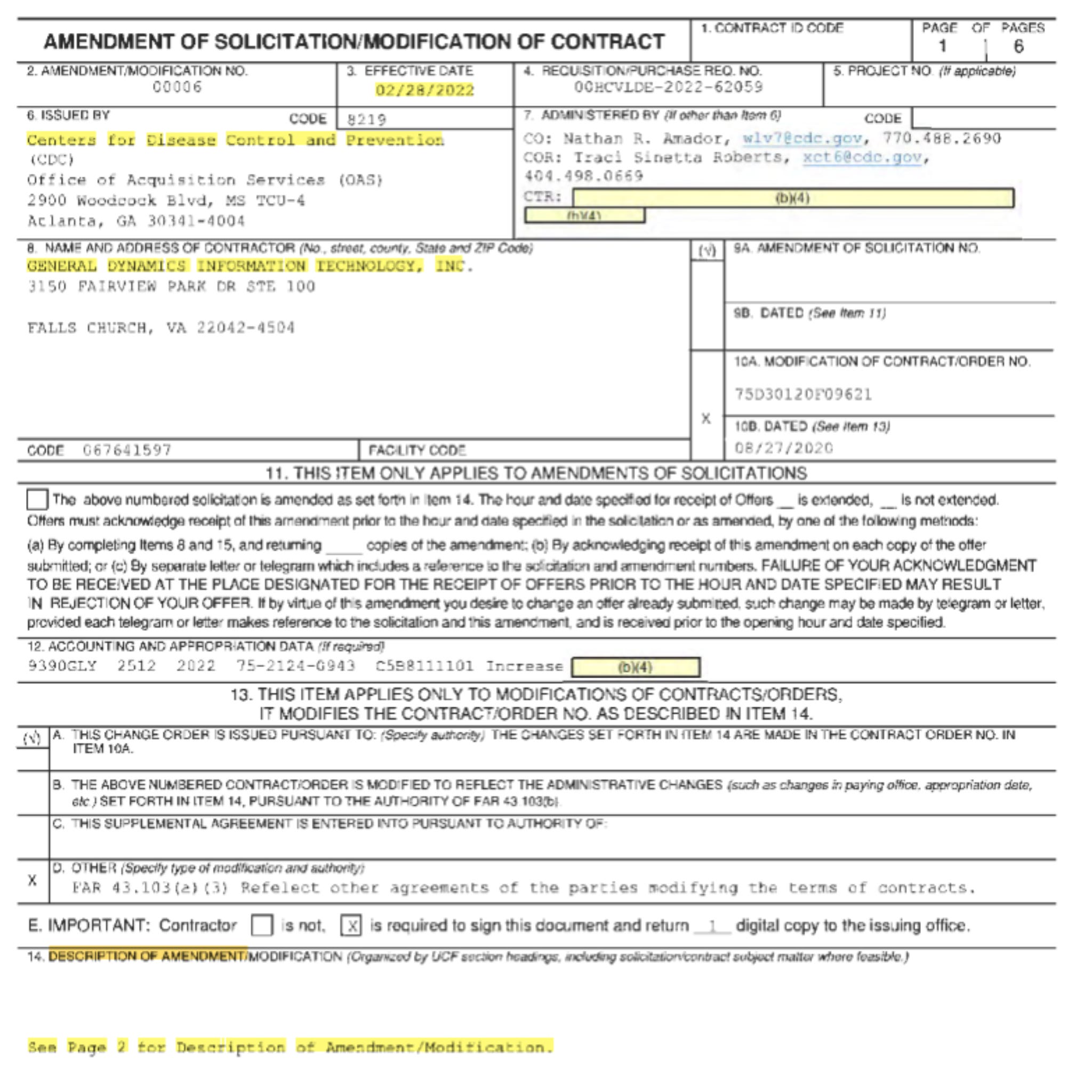

It is unclear whether any new or amended contracts were signed between CDC, FDA, and General Dynamics Information Technology beyond this latest amended contract on February 28, 2022.
Additionally, I discovered that Eagle Health Analytics was awarded a separate contract by the CDC and FDA dated July 8, 2021. As part of this contract, they are seeking assistance with various things, including medical officers and epidemiologist support for the VAERS program.
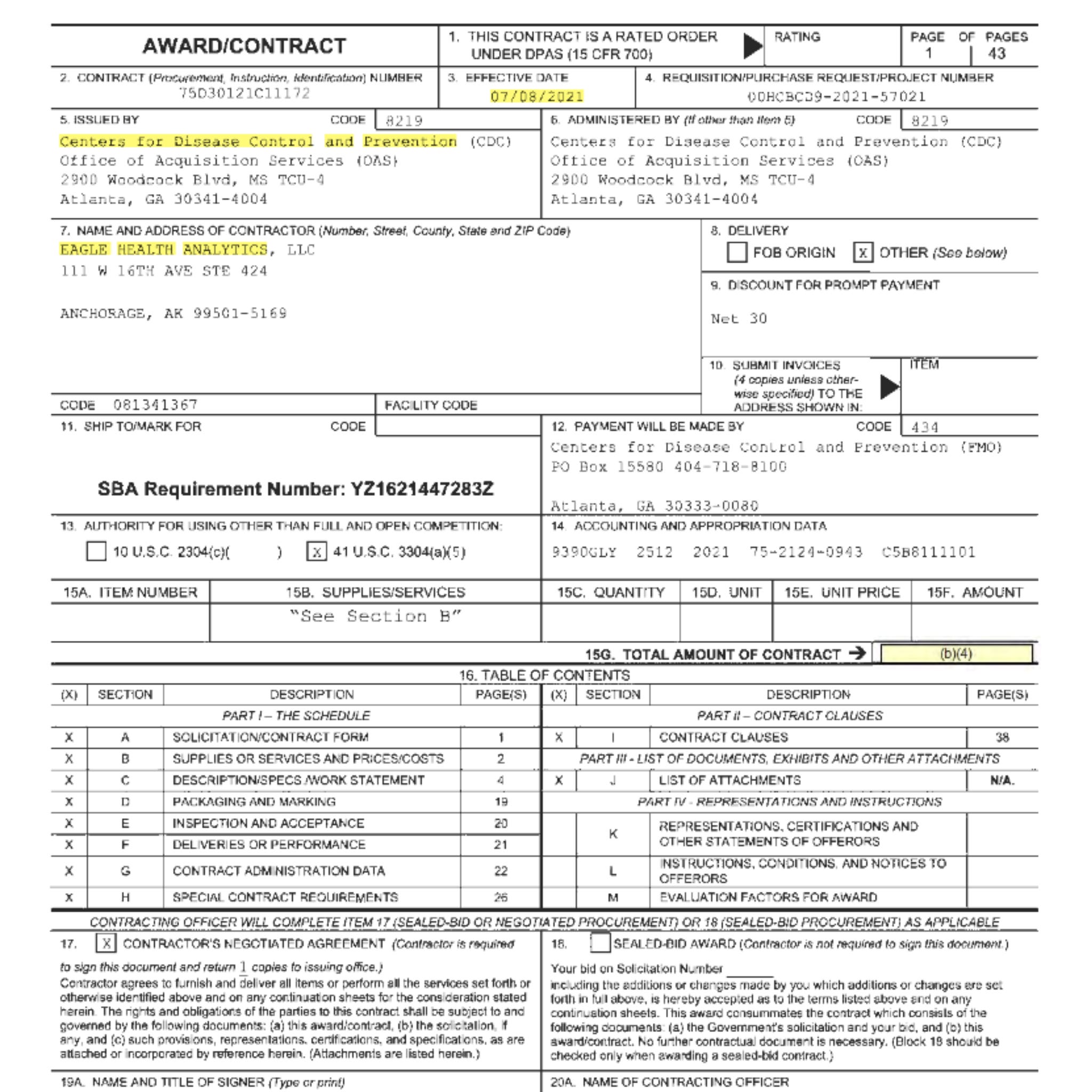
Within the contract on page 5 the CDC says, “VAERS has had a surge in reporting of adverse events as compared to previous years and to other vaccines.”

Over 1.5 million reports of adverse events related to Covid-19 vaccines have been received by the Vaccine Adverse Event Reporting System from December 2020 to March 2023. The reports include just under 35K deaths, almost 200K hospitalizations, over 64K permanent disability, and over 1200 birth defects.
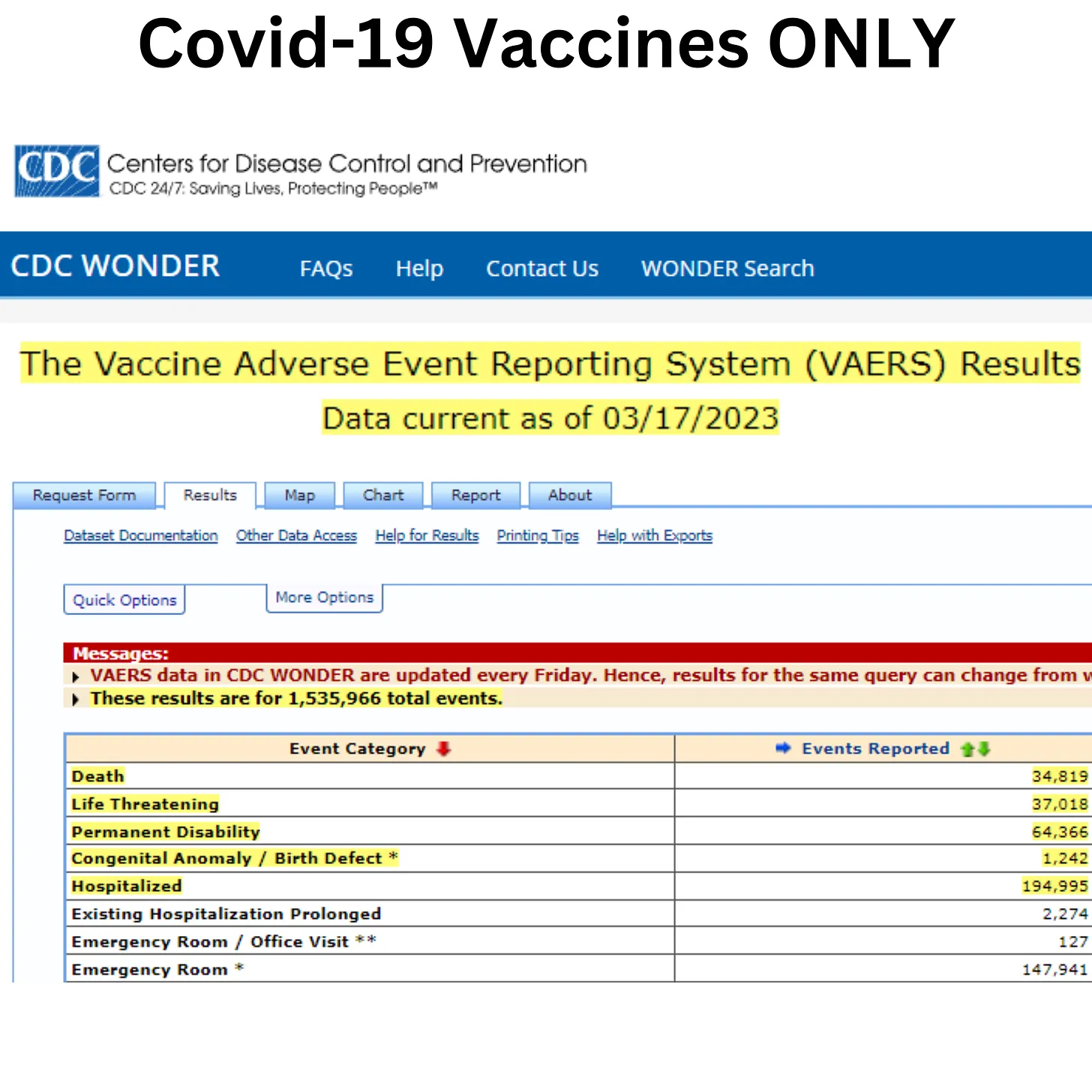
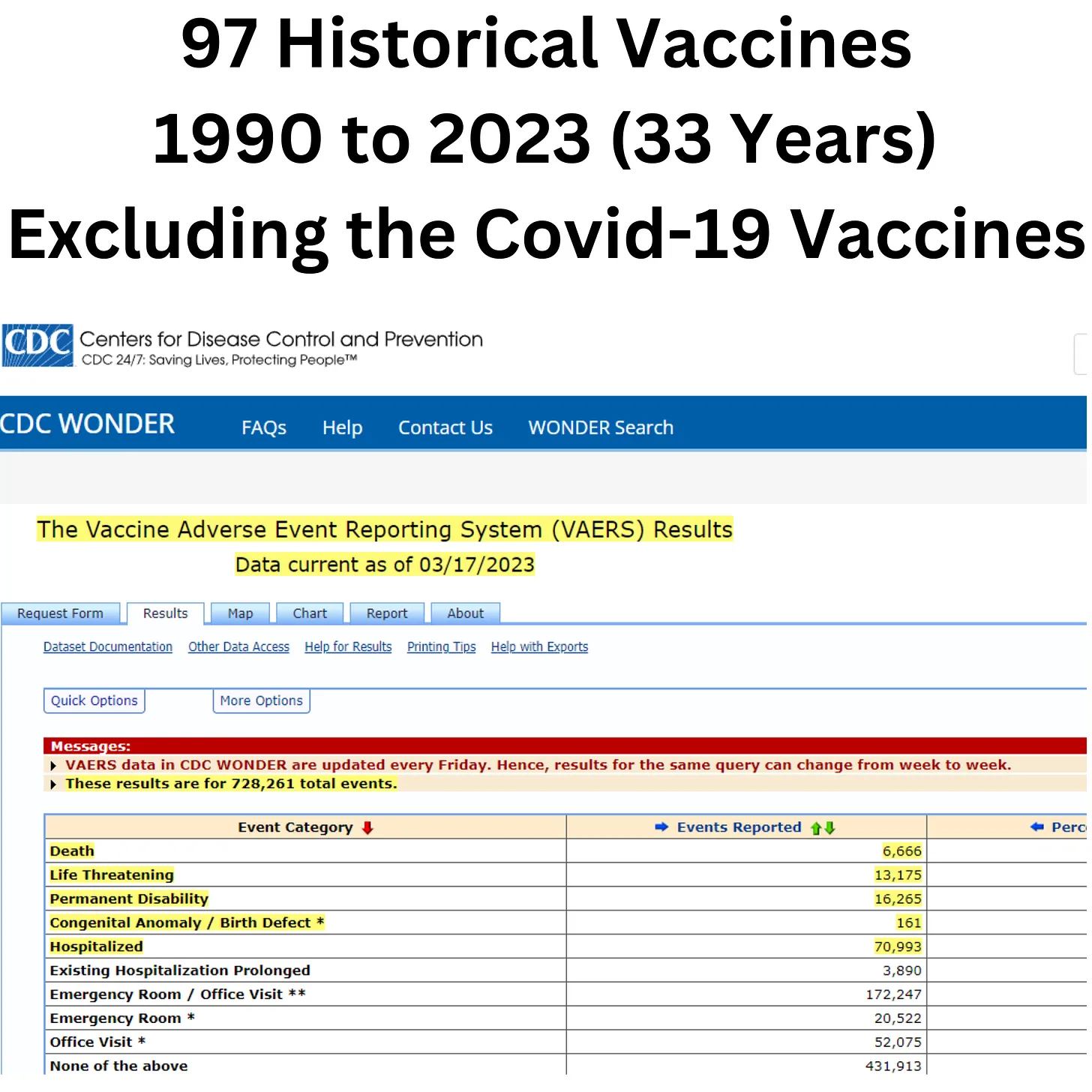
The VAERS data shows the Covid-19 vaccines have extremely higher adverse event reports, including hospitalizations, permanent disability, birth defects, and death, when compared to all other historical vaccines combined over 33 years.
Its worth noting that according to the FDA. “It does not get most reports of adverse events that occur in the United States. Estimates suggest that FDA receives reports of about 1 to 10 percent of the adverse events that occur.”

Originally published on The Canadian Independent
Suggest a correction







![A Call to Europe: The Future of Our Children is at Stake – Historical Press Conf. in Brussels Jan 23 [12 Videos with Subtitles]](https://childrenshealthdefense.eu/wp-content/uploads/2022/01/chdeu-brussels-768x400.jpg)